Reposted with permission from ©AANS, 2014
J Neurosurg Pediatrics (Suppl) 14:53–59, 2014
AANS, 2014
(Used with permission from Journal of Neurosurgery: Pediatrics. Please click here for the original publication.)
Pediatric hydrocephalus: systematic literature review and evidence-based guidelines
Part 7: Antibiotic-impregnated shunt systems versus conventional shunts in children: a systematic review and meta-analysis
UPDATE
Paul Klimo Jr. MD, MPH ,1–3 Clinton J. Thompson, PhD,4 Lissa C. Baird, MD,4 Ann Marie Flannery, MD5
1Semmes-Murphey Neurologic & Spine Institute; 2Department of Neurosurgery, University of Tennessee Health Science Center; 3Le Bonheur Children’s Hospital, Memphis, Tennessee; 4School of Public Health and Health Services, The George Washington University, Washington, DC; and 5Department of Neurological Surgery, Saint Louis University, St. Louis, Missouri
Object. The objective of this systematic review and meta-analysis was to answer the following question: Are antibiotic-impregnated shunts (AISs) superior to standard shunts (SSs) at reducing the risk of shunt infection in pediatric patients with hydrocephalus?
Methods. Both the US National Library of Medicine PubMed/MEDLINE database and the Cochrane Database of Systematic Reviews were queried using MeSH headings and key words relevant to AIS use in children. Abstracts were reviewed, after which studies meeting the inclusion criteria were selected. An evidentiary table was assembled summarizing the studies and the quality of their evidence (Classes I–III). A meta-analysis was conducted using a random-effects model to calculate a cumulative estimate of treatment effect using risk ratio (RR). Heterogeneity was assessed using the chi-square and I2 statistics. Based on the quality of the literature and the result of the meta-analysis, a recommendation was rendered (Level I, II, or III).
Results. Six studies, all Class III, met our inclusion criteria. All but one study focused on a retrospective cohort and all but one were conducted at a single institution. Four of the studies failed to demonstrate a lowered infection rate with the use of an AIS. However, when the data from individual studies were pooled together, the infection rate in the AIS group was 5.5% compared with 8.6% in the SS group. Using a random-effects model, the cumulative RR was 0.51 (95% CI 0.29–0.89, p < 0.001), indicating that a shunt infection was 1.96 times more likely in patients who received an SS.
Conclusions. We recommend AIS tubing because of the associated lower risk of shunt infection compared to the use of conventional silicone hardware (quality of evidence: Class III; strength of recommendation: Level III).
Recommendation: Antibiotic-impregnated shunt (AIS) tubing may be associated with a lower risk of shunt infection compared with conventional silicone hardware and thus is an option for children who require placement of a shunt. Strength of Recommendation: Level III, unclear degree of clinical certainty. (http://thejns.org/doi/abs/10.3171/2014.7.PEDS14327)
Key Words: antibiotic-impregnated shunt, shunt, cerebrospinal fluid, infection, pediatric patient, meta-analysis, hydrocephalus, practice guidelines
Abbreviations used in this paper: AIS = antibiotic-impregnated shunt; AANS = American Association of Neurological Surgeons; CNS = Congress of Neurological Surgeons; RR = risk ratio; SS = standard shunt.
Prevention of shunt infection is a priority for neurosurgeons, especially when treating pediatric patients. Infection can cause shunt malfunction with all the potential consequences of a nonfunctioning shunt. Shunt infection can lead to scarring and loculation of the ventricles, increasing the complexity of the patient’s hydrocephalus, and it may result in a lower intelligence quotient, increased risk of seizures, and psychomotor retardation.1-5 Treatment of shunt infections is costly, estimated to be upwards of $50,000 per infection in the United States, making it one of the most costly implant-related infections.6
The identification of modifiable risk factors or interventions to lower the risk of a shunt infection has been the topic of active research for many years. Identified factors include the duration of surgery;7,8 the skill and experience of the treating neurosurgeon;9-11 the number of personnel in the operating room;12-14 and the use of hair shaving,8,15 prophylactic systemic antibiotics,16-18 intrathecal antibiotics,19 wound irrigation,20 antibiotic-impregnated sutures,21,22 and double gloving23 (or inadvertent exposure of the shunt to breached surgical gloves).24 Antibiotic-impregnated Silastic catheters were first introduced by Roger Bayston in 1977; they were considered more specifically with shunts in 1989,25 but did not become available for clinical use in the United States until about 10 years ago. The antibiotic-impregnated shunt (AIS) systems currently on the market contain 0.054% rifampin and 0.15% clindamycin, which target the most common pathogens: Staphylococcus epidermidis and Staphylococcus aureus. Although rifampin and clindamycin do not reduce bacterial adherence, this combination of antibiotics kills bacteria and has been shown to prevent colonization for up to 56 days in in vitro studies and up to 127 days in vivo.26-28
Many studies have evaluated the efficacy of AISs compared with standard shunts (SSs) in the prevention of shunt infections, including two recent systematic reviews and meta-analyses.29-47 The purpose of this evidence-based review is to examine data on the use of AISs and SSs and compare these treatments in the prevention of shunt infections in the pediatric population.
Methods
Search Terms
We searched the US National Library of Medicine PubMed/MEDLINE database and the Cochrane Database of Systematic Reviews for the period January 1966 through March 2012 using the following MeSH subject headings: (“cerebrospinal fluid shunts” OR (“cerebro- spinal fluid” AND (shunt* OR catheter*)) OR “shunt system”) AND (“antibiotic-impregnated” OR (antibiotic AND impregnated)) AND infection.
Search Strategy
We reviewed the titles and abstracts of the papers we retrieved with attention to those titles addressing the rate of shunt infection in patients treated with AISs compared with those treated with SSs. Uncontrolled studies were excluded, as were studies that evaluated antimicrobial shunts unavailable in the US market. In all papers, we required that the authors state that the only variable that changed was the type of shunt implanted; all other aspects of the surgery and technique needed to remain unchanged.
Authors performed an updated literature search (in PubMed and Cochrane Central) for this guideline chapter through a medical librarian at the Congress of Neurological Surgeons Guidelines office using the below-mentioned existing search terms to update the original search through November 30, 2019.
Meta-Analysis
For each study, we identified the number of infections resulting from implantation of SSs and AISs and then computed the risk of an infection associated with AISs relative to that associated with SSs, yielding a risk ratio (RR). An RR less than 1 is indicative of protection against infection for the AIS. The overall RR was computed using the method of DerSimonian and Laird.48
We conducted a random-effects meta-analysis of the selected studies. A random-effects model—as opposed to a fixed-effects model—does not assume that the measure of association (that is, RR) is uniform across strata (that is, among studies) and, consequently, yields a more conservative estimate of the effect. We assessed heterogeneity by way of the chi-square test of heterogeneity and the I2 statistic, in which the former returns a chi-square distributed test statistic and corresponding p value and the latter returns a value bound between 0% and 100%, with higher values denoting increasing heterogeneity. We regarded a chi-square test of heterogeneity p value less than alpha = 0.10 and an I2 value in the range of 30% to 60% as suggestive of moderate heterogeneity.49,50 An examination of publication bias was not conducted since the number of studies included in this analysis was not large enough to provide adequate power (i.e., fewer than 10 studies).
Search Results
Our search returned 41 articles; another 3 articles were found from an examination of the articles’ bibliographies (Fig. 1). Nineteen full-length papers were reviewed, 13 of which were rejected for the following reasons: studies enrolled either adults only or enrolled mixed populations, but separate results for children were not provided,29,33,34,41-44,46 or studies contained patient data that had also been reported in separate publications.21,51-54 In fact, 1 group of researchers published no less than 9 papers on AISs that included patients from overlapping time periods.21,32,40,41,45,51,53-55 Therefore, 6 articles satisfied inclusion for this systematic review and meta-analysis (Table 1).30,31,36-38,45
An additional 5 studies out of the 122 yielded by the 2020 update met inclusion criteria from the original guideline and were included (Figure 2).
Results
The review process identified no papers providing Class I or II data specifically addressing the issue of shunt infection and the use of AISs compared with SSs in children. The 6 articles that satisfied our entry criteria were all Class III cohort studies, all but one of which were conducted within a single institution. The primary outcome of interest—shunt infection—was defined by authors of individual studies, but in general, it was a patient who underwent a recent shunt surgery and subsequently developed signs and symptoms of a shunt malfunction or an infection with an organism cultured from CSF, the shunt apparatus, purulence from the shunt wound(s), or abdominal fluid/pseudocyst. Some investigators also considered a patient to have an infection if there were highly suggestive findings such as fever, redness along the shunt, or CSF pleocytosis in the absence of a positive culture. Overall, 2 studies produced findings that AISs are protective against shunt infection, whereas the remaining studies did not.
Sciubba et al45 reported one of the earliest large series comparing AISs with SSs in a pediatric population. During an 18-month period, 208 SSs were placed; this was followed by another 18-month period during which AISs were used 145 times. The AIS patient group was younger, more frequently premature, and thus had a greater incidence of intracranial hemorrhage as the cause of hydrocephalus. The primary outcome was the development of a shunt infection, defined as clinical suspicion (fever, increased white blood cell count, and/or wound breakdown involving the shunt) with positive cultures from CSF and/or hardware. Patients who received AIS catheters had significantly fewer shunt infections: 2 patients (1.4%) with antibiotic-impregnated catheters within the 6-month follow-up period compared with 25 patients (12%) with non–antibiotic-impregnated catheters. After we adjusted for intercohort differences in primary placement compared with shunt revision, prematurity, and posthemorrhagic hydrocephalus, we found AIS catheters to be independently associated with a 2.4-fold decreased likelihood of shunt infection.
Aryan et al30 detailed their 1-year experience using the Bactiseal system (Codman, Johnson & Johnson). Al- though the rate of shunt infection was lower in the Bactiseal group (1 of 32 [3.1%]) compared with the standard group (7 of 46 [15.2%]), the difference was not statistically significant (p = 0.09). Kan and Kestler37 reported on a similar retrospective cohort in which 80 consecutive patients received the Bactiseal shunt and were compared with an earlier group of 80 patients who had received an SS. There was no statistically significant difference in the shunt infection rate (5.0% vs 8.8%), even when the authors controlled for patient age at surgery, type of revision, cause of hydrocephalus, and previous revisions or infections within the past 6 months.
In their retrospective cohort study, Hayhurst and co-workers36 looked at 4 groups of patients in whom AISs had been implanted de novo (Group 1), during noninfected revisional surgery (Group 2), and after an external ventricular drain had been replaced by the shunt (sterile CSF [Group 3] and infected CSF [Group 4]). There were 214 shunt procedures performed using the Bactiseal system in 150 children. The historical control group comprised 77 operations in 65 children. Again, there was no statistically significant difference in the infection rate (21 of 214 [9.8%] in the antibiotic group and 8 of 77 [10.4%] in the standard group). Although the authors emphasized the difference in the infection rate among neonates—27% in the standard group versus 11% in the antibiotic group— this difference too was not significant (p = 0.208). Eymann et al31 presented clinical and cost data for both adult and pediatric patients. Using Fisher’s exact test, the pediatric infection rates of 13.6% in the standard group and 3.8% in the Bactiseal group were not statistically different. However, when the authors combined both adult and pediatric outcomes, they did find a protective benefit with the Bactiseal system and a net savings of $51,651 in the 197 Bactiseal procedures.
The study with the largest number of patients was conducted by Kandasamy et al.38 This multicenter study (3 pediatric neurosurgery centers in the United Kingdom) was ambispective: patients treated with AISs were prospectively followed, whereas patients treated with SSs at earlier time periods were retrospectively reviewed (historical control). Operations were divided into those that were de novo and those that were clean revisions. There was some intercenter variability in the choice of preoperative antibiotics and surgical technique, but there was no intracenter variability. For example, centers at Leeds and Liverpool used a single dose of cefuroxime, whereas London used flucloxacillin and amikacin. The overall pooled treatment effect estimate statistically favored AISs for de novo and clean revisions combined (the incidence of infection in the AIS Group was 30 of 581 [5.2%] and that in the SS Group was 155 of 1963 [7.9%]) as well as for the subgroup of de novo shunts only and the subgroup of children younger than 1 year of age; the pooled treatment effect estimate for clean revisions only did not reach statistical significance.
Meta-Analysis Results
In total, there were 2396 procedures in which a standard catheter system had been placed and 205 infections occurred, yielding a pooled infection rate of 8.6%. In the AIS population, 59 infections occurred after 1078 shunt operations for an overall infection rate of 5.5%. Thus, the absolute and relative risk reductions were 3.1% and 36%, respectively. The overall RR was 0.51 (95% CI 0.29–0.89, p < 0.001), making a shunt infection 1.96 times more likely when an SS system is used (Fig. 2). Although the chi- square test did not indicate heterogeneity (p = 0.129), the I2 test did show moderate heterogeneity (41.5%).
To explore the uncertainty of statistical significance in the RR meta-analysis, a stepwise sensitivity analysis was performed (Table 2). When subtracting studies from the meta-analysis (and thus reducing the power of the analysis), the effect size remains relatively stable, but confidence intervals widen to the point of statistical nonsignificance. Based on significant findings in large studies comparing AISs with SSs and significant findings in a meta-analysis with a high number of studies, it is likely that the meta- analysis shown in Fig. 2 accurately represents a statistically significant effect in favor of using AISs.
Number Needed to Treat
There is a certain difficulty with interpreting an RR of 0.51, in that the number of people who benefited from AIS treatment is masked by the interpretation of an RR (i.e., a “50% reduced risk of infection”). In fact the infection rate in the AIS patient group in this meta-analysis was 5.47% compared with 8.55% in the SS patient group. These infection rates come close to approximating a “50% reduced risk of infection.”
To better understand the analysis of AISs versus SSs, absolute values calculated as the number of cases needed to treat and the number of infections avoided per 1000 cases treated with AISs were calculated (Table 2). According to the data reported in the literature, for every 24 cases treated with an AIS, 1 infection is prevented. Alternatively, 42 infections are avoided for every 1000 cases treated with AISs. As a convenience, several population infection rates (that is, infection rates unique to particular locations/practices) are presented in Table 3. Not surprisingly, the higher an infection rate in a population, the “better” the AIS becomes at preventing infection.
2020 Update
Mbabazi et al56, which was a single center RCT, provided Class II evidence that in a unique group of patients (low resource country), single center, a unique antibiotic impregnated catheter design (distal slit valve) failed to show an advantage in infection prevention. Because the population in this study is so unique, the authors concluded that this study, while meeting inclusion criteria, does not affect the recommendation. Three other publications57-59 were single center, retrospective reviews, Class III. Mallucci et al60 conducted a multicenter Class I RCT of both adult and pediatric patients, and demonstrated that antibiotic impregnated shunts do reduce the rate of infection, particularly in children. This new evidence warrants an upgrade of the level of the recommendation for this chapter.
Conclusions
Recommendation: Antibiotic-impregnated shunt (AIS) tubing reduces the risk of shunt infection compared with conventional silicone hardware and should be used for children who require placement of a shunt. Strength of Recommendation: Level I, high degree of clinical certainty.
The clinical and financial consequences of a shunt infection are substantial as is the emotional stress borne by patients and their families. Neurosurgeons have evaluated many interventions in the hopes of finding ones that can decrease the risk of developing a shunt infection. Based on the available Class III evidence, we have demonstrated that antibiotic-impregnated shunts (AISs containing rifampin and clindamycin) can lower the shunt infection risk substantially. Although only 2 of the 6 studies that met our inclusion criteria showed a protective benefit with AISs, when the data from all 6 studies were pooled together (meta-analysis), a benefit was shown, with an infection rate almost twice as high in patients receiving a standard shunt (SS). Given the large number of patients that would be needed to definitively demonstrate superior efficacy of AISs over SSs in children, it is unlikely that a clinical trial will be conducted or is even needed.
Acknowledgments
We acknowledge the American Association of Neurological Surgeons (AANS)/Congress of Neurological Surgeons (CNS) Joint Guidelines Committee for the members’ reviews, comments, and suggestions; Laura Mitchell, Guidelines Project Manager for the CNS, for her contributions; Pamela Shaw, research librarian, for her assistance with the literature searches; Kevin Boyer for his assistance with data analysis; and Sue Ann Kawecki and Kristin Kraus, M.Sc., for their assistance with editing. We also acknowledge the following peer reviewers for their contributions to review the update to the guidelines: Jennifer Sweet, MD, Brandon Rocque, MD, Christoph Greissenauer, MD, Jeffrey Olson, MD.
Disclosure
The systematic review and evidence-based guidelines were funded exclusively by the CNS and AANS Pediatric Section, which received no funding from outside commercial sources to support the development of this document.
Conflict(s) of Interest: None. All Task Force members declared any potential conflicts of interest prior to beginning work on this evidence review.
Conflict(s) of Interest: None. All Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Update Task Force members declared any potential conflicts of interest prior to beginning work on this systematic review and evidence-based guidelines.
Author contributions to the study and manuscript preparation include the following. Conception and design: AANS/CNS Joint Section on Pediatrics. Acquisition of data: all authors. Analysis and interpretation of data: all authors. Drafting the article: Klimo. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Flannery. Statistical analysis: all authors. Administrative/technical/material support: all authors. Study supervision: Flannery.
References
- Chadduck W, Adametz J. Incidence of seizures in patients with myelomeningocele: a multifactorial analysis. Surg Neurol. 1988;30(4):281-285.
- Jamjoom AB, Mohammed AA, al-Boukai A, Jamjoom ZA, Rahman N, Jamjoom HT. Multiloculated hydrocephalus related to cerebrospinal fluid shunt infection. Acta neurochirurgica. 1996;138(6):714-719.
- Mapstone TB, Rekate HL, Nulsen FE, Dixon MS, Jr., Glaser N, Jaffe M. Relationship of CSF shunting and IQ in children with myelomeningocele: a retrospective analysis. Child's brain. 1984;11(2):112-118.
- Vanaclocha V, Saiz-Sapena N, Leiva J. Shunt malfunction in relation to shunt infection. Acta neurochirurgica. 1996;138(7):829-834.
- Walters BC, Hoffman HJ, Hendrick EB, Humphreys RP. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. Journal of neurosurgery. 1984;60(5):1014-1021.
- Darouiche RO. Treatment of infections associated with surgical implants. The New England journal of medicine. 2004;350(14):1422-1429.
- Kontny U, Hofling B, Gutjahr P, Voth D, Schwarz M, Schmitt HJ. CSF shunt infections in children. Infection. 1993;21(2):89-92.
- Ratanalert S, Musikawat P, Oearsakul T, Saeheng S, Chowchuvech V. Non-shaved ventriculoperitoneal shunt in Thailand. In: J Clin Neurosci. Vol 12. Scotland2005:147-149.
- Borgbjerg BM, Gjerris F, Albeck MJ, Borgesen SE. Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first-time shunts. Acta neurochirurgica. 1995;136(1-2):1-7.
- Cochrane DD, Kestle JR. The influence of surgical operative experience on the duration of first ventriculoperitoneal shunt function and infection. In: Pediatr Neurosurg. Vol 38. Switzerland: 2003 S. Karger AG, Basel; 2003:295-301.
- Kestle JR, Cochrane DD, Drake JM. Shunt insertion in the summer: is it safe? Journal of neurosurgery. 2006;105(3 Suppl):165-168.
- Choux M, Genitori L, Lang D, Lena G. Shunt implantation: reducing the incidence of shunt infection. Journal of neurosurgery. 1992;77(6):875-880.
- Kestle JR, Hoffman HJ, Soloniuk D, Humphreys RP, Drake JM, Hendrick EB. A concerted effort to prevent shunt infection. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1993;9(3):163-165.
- Pirotte BJ, Lubansu A, Bruneau M, Loqa C, Van Cutsem N, Brotchi J. Sterile surgical technique for shunt placement reduces the shunt infection rate in children: preliminary analysis of a prospective protocol in 115 consecutive procedures. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2007;23(11):1251-1261.
- Horgan MA, Piatt JH, Jr. Shaving of the scalp may increase the rate of infection in CSF shunt surgery. Pediatric neurosurgery. 1997;26(4):180-184.
- Haines SJ, Walters BC. Antibiotic prophylaxis for cerebrospinal fluid shunts: a metanalysis. Neurosurgery. 1994;34(1):87-92.
- Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts: a systematic review. Journal of neurosurgery Pediatrics. 2008;1(1):48-56.
- Zentner J, Gilsbach J, Felder T. Antibiotic prophylaxis in cerebrospinal fluid shunting: a prospective randomized trial in 129 patients. Neurosurgical review. 1995;18(3):169-172.
- Ragel BT, Browd SR, Schmidt RH. Surgical shunt infection: significant reduction when using intraventricular and systemic antibiotic agents. Journal of neurosurgery. 2006;105(2):242-247.
- Hayashi T, Shirane R, Kato T, Tominaga T. Efficacy of intraoperative wound irrigation for preventing shunt infection. Journal of neurosurgery Pediatrics. 2008;2(1):25-28.
- Sciubba DM, Lin LM, Woodworth GF, McGirt MJ, Carson B, Jallo GI. Factors contributing to the medical costs of cerebrospinal fluid shunt infection treatment in pediatric patients with standard shunt components compared with those in patients with antibiotic impregnated components. In: Neurosurg Focus. Vol 22. United States2007:E9.
- Stone J, Gruber TJ, Rozzelle CJ. Healthcare savings associated with reduced infection rates using antimicrobial suture wound closure for cerebrospinal fluid shunt procedures. In: Pediatr Neurosurg. Vol 46. Switzerland: 2010 S. Karger AG, Basel.; 2010:19-24.
- Rehman AU, Rehman TU, Bashir HH, Gupta V. A simple method to reduce infection of ventriculoperitoneal shunts. Journal of neurosurgery Pediatrics. 2010;5(6):569-572.
- Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: a prospective study of risk factors. Journal of neurosurgery. 2001;94(2):195-201.
- Bayston R, Grove N, Siegel J, Lawellin D, Barsham S. Prevention of hydrocephalus shunt catheter colonisation in vitro by impregnation with antimicrobials. Journal of neurology, neurosurgery, and psychiatry. 1989;52(5):605-609.
- Bayston R, Ashraf W, Bhundia C. Mode of action of an antimicrobial biomaterial for use in hydrocephalus shunts. In: J Antimicrob Chemother. Vol 53. England2004:778-782.
- Bayston R, Lambert E. Duration of protective activity of cerebrospinal fluid shunt catheters impregnated with antimicrobial agents to prevent bacterial catheter-related infection. Journal of neurosurgery. 1997;87(2):247-251.
- Pattavilakom A, Kotasnas D, Korman TM, Xenos C, Danks A. Duration of in vivo antimicrobial activity of antibiotic-impregnated cerebrospinal fluid catheters. In: Neurosurgery. Vol 58. United States2006:930-935; discussion 930-935.
- Albanese A, De Bonis P, Sabatino G, et al. Antibiotic-impregnated ventriculo-peritoneal shunts in patients at high risk of infection. Acta neurochirurgica. 2009;151(10):1259-1263.
- Aryan HE, Meltzer HS, Park MS, Bennett RL, Jandial R, Levy ML. Initial experience with antibiotic-impregnated silicone catheters for shunting of cerebrospinal fluid in children. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2005;21(1):56-61.
- Eymann R, Chehab S, Strowitzki M, Steudel WI, Kiefer M. Clinical and economic consequences of antibiotic-impregnated cerebrospinal fluid shunt catheters. Journal of neurosurgery Pediatrics. 2008;1(6):444-450.
- Farber SH, Parker SL, Adogwa O, McGirt MJ, Rigamonti D. Effect of antibiotic impregnated shunts on infection rate in adult hydrocephalus: A single institution's experience. Neurosurgery. 2011.
- Govender ST, Nathoo N, van Dellen JR. Evaluation of an antibiotic-impregnated shunt system for the treatment of hydrocephalus. Journal of neurosurgery. 2003;99(5):831-839.
- Gutierrez-Gonzalez R, Boto GR. Do antibiotic-impregnated catheters prevent infection in CSF diversion procedures? Review of the literature. The Journal of infection. 2010;61(1):9-20.
- Gutierrez-Gonzalez R, Boto GR, Fernandez-Perez C, del Prado N. Protective effect of rifampicin and clindamycin impregnated devices against Staphylococcus spp. infection after cerebrospinal fluid diversion procedures. In: BMC Neurol. Vol 10. England2010:93.
- Hayhurst C, Cooke R, Williams D, Kandasamy J, O'Brien DF, Mallucci CL. The impact of antibiotic-impregnated catheters on shunt infection in children and neonates. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2008;24(5):557-562.
- Kan P, Kestle J. Lack of efficacy of antibiotic-impregnated shunt systems in preventing shunt infections in children. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2007;23(7):773-777.
- Kandasamy J, Dwan K, Hartley JC, et al. Antibiotic-impregnated ventriculoperitoneal shunts-a multi-centre British paediatric neurosurgery group (BPNG) study using historical controls. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2010.
- Klimo P, Jr., Thompson CJ, Ragel BT, Boop FA. Antibiotic-impregnated shunt systems versus standard shunt systems: a meta- and cost-savings analysis. Journal of neurosurgery Pediatrics. 2011;8(6):600-612.
- Parker SL, Anderson WN, Lilienfeld S, Megerian JT, McGirt MJ. Cerebrospinal shunt infection in patients receiving antibiotic-impregnated versus standard shunts. Journal of neurosurgery Pediatrics. 2011;8(3):259-265.
- Parker SL, Attenello FJ, Sciubba DM, et al. Comparison of shunt infection incidence in high-risk subgroups receiving antibiotic-impregnated versus standard shunts. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2009;25(1):77-83; discussion 85.
- Pattavilakom A, Xenos C, Bradfield O, Danks RA. Reduction in shunt infection using antibiotic impregnated CSF shunt catheters: an Australian prospective study. In: J Clin Neurosci. Vol 14. Scotland2007:526-531.
- Richards HK, Seeley HM, Pickard JD. Efficacy of antibiotic-impregnated shunt catheters in reducing shunt infection: data from the United Kingdom Shunt Registry. Journal of neurosurgery Pediatrics. 2009;4(4):389-393.
- Ritz R, Roser F, Morgalla M, Dietz K, Tatagiba M, Will BE. Do antibiotic-impregnated shunts in hydrocephalus therapy reduce the risk of infection? An observational study in 258 patients. In: BMC Infect Dis. Vol 7. England2007:38.
- Sciubba DM, Stuart RM, McGirt MJ, et al. Effect of antibiotic-impregnated shunt catheters in decreasing the incidence of shunt infection in the treatment of hydrocephalus. Journal of neurosurgery. 2005;103(2 Suppl):131-136.
- Steinbok P, Milner R, Agrawal D, et al. A multicenter multinational registry for assessing ventriculoperitoneal shunt infections for hydrocephalus. Neurosurgery. 2010;67(5):1303-1310.
- Thomas R, Lee S, Patole S, Rao S. Antibiotic-impregnated catheters for the prevention of CSF shunt infections: a systematic review and meta-analysis. British journal of neurosurgery. 2012;26(2):175-184.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
- Crowther M, Lim W, Crowther MA. Systematic review and meta-analysis methodology. Blood. 2010;116(17):3140-3146.
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions 2011; 5.1.0:www.cochrane-handbook.org.
- Attenello FJ, Garces-Ambrossi GL, Zaidi HA, Sciubba DM, Jallo GI. Hospital costs associated with shunt infections in patients receiving antibiotic-impregnated shunt catheters versus standard shunt catheters. Neurosurgery. 2010;66(2):284-289; discussion 289.
- Eymann R, Steudel WI, Kiefer M. Infection rate with application of an antibiotic-impregnated catheter for shunt implantation in children - a retrospective analysis. Klinische Padiatrie. 2009;221(2):69-73.
- Sciubba DM, McGirt MJ, Woodworth GF, Carson B, Jallo GI. Prolonged exposure to antibiotic-impregnated shunt catheters does not increase incidence of late shunt infections. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2007;23(8):867-871.
- Sciubba DM, Noggle JC, Carson BS, Jallo GI. Antibiotic-impregnated shunt catheters for the treatment of infantile hydrocephalus. In: Pediatr Neurosurg. Vol 44. Switzerland2008:91-96.
- Farber SH, Parker SL, Adogwa O, Rigamonti D, McGirt MJ. Cost analysis of antibiotic-impregnated catheters in the treatment of hydrocephalus in adult patients. World neurosurgery. 2010;74(4-5):528-531.
- Mbabazi-Kabachelor E, Shah M, Vaughan KA, et al. Infection risk for Bactiseal Universal Shunts versus Chhabra shunts in Ugandan infants: a randomized controlled trial. Journal of neurosurgery Pediatrics. 2019;23(3):397-406.
- Raffa G, Marseglia L, Gitto E, Germano A. Antibiotic-impregnated catheters reduce ventriculoperitoneal shunt infection rate in high-risk newborns and infants. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2015;31(7):1129-1138.
- Lane JD, Mugamba J, Ssenyonga P, Warf BC. Effectiveness of the Bactiseal Universal Shunt for reducing shunt infection in a sub-Saharan African context: a retrospective cohort study in 160 Ugandan children. Journal of neurosurgery Pediatrics. 2014;13(2):140-144.
- Jaeger W, Lee S, Vineet D, Keil A, Agarwal N, Rao S. Ventriculoperitoneal shunts in neonates: a retrospective study of outcomes with antibiotic-impregnated catheters and a modified peri-operative antibiotic protocol. British journal of neurosurgery. 2017;31(6):672-676.
- Mallucci CL, Jenkinson MD, Conroy EJ, et al. Antibiotic or silver versus standard ventriculoperitoneal shunts (BASICS): a multicentre, single-blinded, randomised trial and economic evaluation. Lancet (London, England). 2019;394(10208):1530-1539.
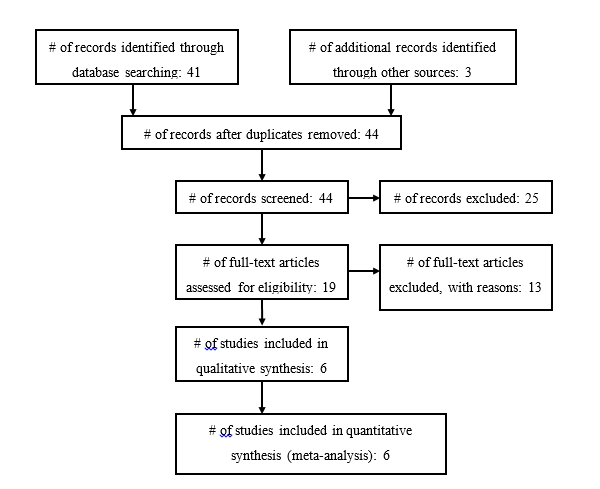
Figure 1a. Included and Excluded Articles Flowchart
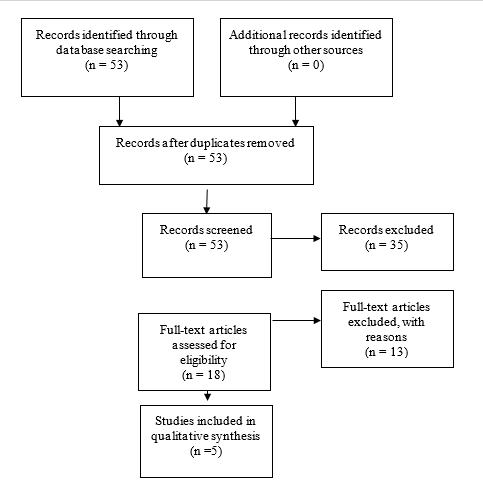
Fig. 1b. Flowchart showing the process involved in identifying relevant literature for the 2020 Update. The criteria for “records excluded” and “full text articles excluded with reasons” are detailed in Part 1 of the Guidelines.
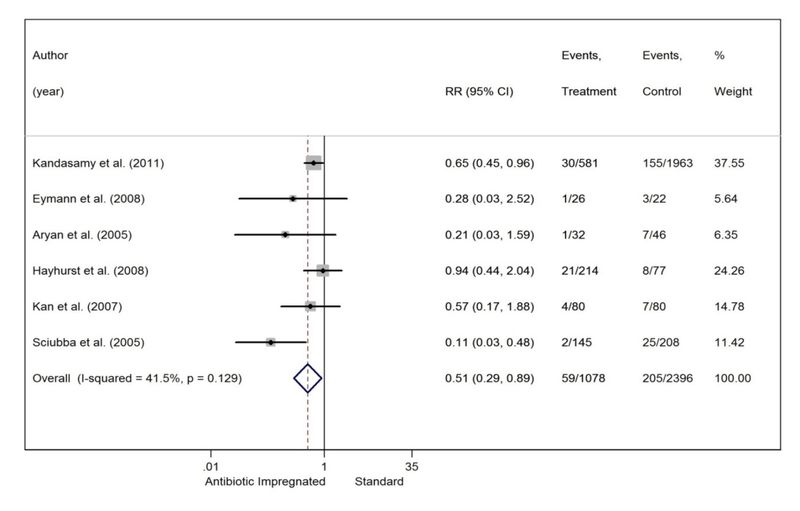
Fig. 2. Forest plot comparing AISs and SSs.
Evidence Tables
Table 1 Antibiotic Impregnated Shunt Systems vs. Conventional Shunts
| Author |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
Kandasamy et al., 2011
|
ambispective, multiinstitution
|
Class III - Ambispective cohort with historical controls used
|
AIS: 30/581 (5.2%); SS: 155/1963 (7.9%). AIS reduced shunt infection rate. |
|
Eymann et al., 2008
|
retrospective, single institution |
Class II - retrospective cohort
|
AIS: 1/26 (3.8%); SS: 3/22 (13.6%). No statistically significant difference.* |
|
|
Aryan et al. 2005
|
retrospective, single institution
|
Class II - retrospective cohort
|
AIS: 1/32 (3.1%); SS: 7/46 (15.2%). No statistically significant difference. |
|
|
Hayhurst et al., 2008
|
retrospective, single institution
|
Class III - historical controls used
|
AIS: 21/214 (9.8%); SS: 8/77 (10.4%). No statistically significant difference. |
|
Kan et al., 2007
|
retrospective, single institution
|
Class II - retrospective cohort
|
AIS: 4/80 (5%); SS: 7/80 (8.8%). No statistically significant difference. |
|
Sciubba et al. 2005
|
retrospective, single institution
|
Class II - retrospective cohort
|
AIS: 2/145 (1.4%); SS: 25/208 (12%). AIS reduced shunt infection rate. |
|
† - percentages are per shunt procedure, not per patient.
*- Fisher’s exact test used.
Table 2 Sensitivity Analysis Results
| N largest studies |
Risk Ratio (95% CI) |
Heterogeneity (I2) |
Result |
|
All studies (Figure 1)
|
0.51 (0.29, 0.89)
|
41.5%
|
Statistically Significant |
|
5 largest studies
|
0.52 (0.28, 0.95) |
50.5%
|
Statistically Significant |
|
|
4 largest studies
|
0.55 (0.29, 1.05)
|
56.5%
|
Not Significant |
|
|
3 largest studies
|
0.52 (0.23, 1.20)
|
71.0%
|
Not Significant |
|
2 largest studies
|
0.31 (0.06, 1.75)
|
82.1%
|
Not Significant |
|
Table 3 Number Needed to Treat
|
Assumed population Infection Rate
|
Number Needed to Treat
|
Number of Infections avoided per 1000 treated with AIS (95% CI) |
|
5%
|
41
|
24 (5, 35)
|
|
|
8.6%*
|
24 |
42 (9, 60)
|
|
|
|
10%
|
21
|
49 (11, 70)
|
|
|
|
12.5%
|
17
|
61 (14, 88)
|
|
|
15%
|
14
|
73 (16, 106)
|
|
|
Table 4. New evidence included in 2020 Update
| Author |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
Mbabazi et al, 2019
|
Single center, prospective RCT that attempts to determine the efficacy of bacteriseal universal shunts in Uganda patients.
|
II
|
In a unique group of patients (low resource country), a unique antibiotic impregnated catheter design (distal slit valve) failed to show an advantage in infection prevention. |
|
Raffa et al, 2015
|
Single center, retrospective review, comparing children who received AIS shunts and those who did not. |
III
|
AISs reduced shunt infection in high-risk pediatric patients > 1 year old. |
|
|
Lane et al, 2014
|
Single center, retrospective review, comparing the efficacy of a Bactiseal shunt system to a non-antibiotic-impregnated system.
|
III
|
No significant different between groups. |
|
|
Jaeger et al, 2017
|
Single center, retrospective review evaluating the use of AIS shunts to decrease neonatal VP shunt infections.
|
III
|
AIS catheters and perioperative antibiotics may be helpful in neonatal hydrocephalus |
|
Mallucci et al, 2019
|
Multicenter, single-blinded RCT comparing antibiotic or silver to standard ventriculoperitoneal shunts (BASICS).
|
I
|
Antibiotic impregnated shunts reduce the rate of infection, particularly in children. |
|