Reposted with permission from ©AANS, 2014
J Neurosurg Pediatrics (Suppl) 14:8–23, 2014
AANS, 2014
(Original text of the guideline was edited to reflect the update. Please click here for the original publication.)
Pediatric hydrocephalus: systematic literature review and evidence-based guidelines
Part 2: Management of posthemorrhagic hydrocephalus in premature infants
UPDATE
Catherine A. Mazzola, MD1, Asim F. Choudhri MD2,3, Kurtus I. Auguste, MD4, David D. Limbrick Jr., MD, PhD5, Marta Rogido, MD6, Laura Mitchell, MA7, Ann Marie Flannery, MD8
1Division of Pediatric Neurological Surgery, Goryeb Children’s Hospital, Morristown, New Jersey; 2Departments of Radiology and Neurosurgery, University of Tennessee Health Science Center, and 3Le Bonheur Neuroscience Institute, Le Bonheur Children’s Hospital, Memphis, Tennessee; 4Department of Neurosurgery, University of California, San Francisco, California; 5Division of Pediatric Neurosurgery, St. Louis Children’s Hospital, St. Louis, Missouri; 6Division of Neonatology, Department of Pediatrics, Goryeb Children’s Hospital, Morristown; and Rutgers New Jersey Medical School, Newark, New Jersey; 7Congress of Neurological Surgeons, Schaumburg, Illinois; and 8Department of Neurological Surgery, Saint Louis University, St. Louis, Missouri
Object. The objective of this systematic review and analysis was to answer the following question: What are the optimal treatment strategies for posthemorrhagic hydrocephalus (PHH) in premature infants?
Methods. Both the US National Library of Medicine and the Cochrane Database of Systematic Reviews were queried using MeSH headings and key words relevant to PHH. Two hundred thirteen abstracts were reviewed, after which 98 full-text publications that met inclusion criteria that had been determined a priori were selected and reviewed.
Results. Following a review process and an evidentiary analysis, 68 full-text articles were accepted for the evidentiary table and 30 publications were rejected. The evidentiary table was assembled linking recommendations to strength of evidence (Classes I–III).
Conclusions. There are 7 recommendations for the management of PHH in infants. Three recommendations reached Level I strength, which represents the highest degree of clinical certainty. There were two Level II and two Level III recommendations for the management of PHH.
Recommendation concerning Surgical temporizing measures: I. Ventricular access devices (VADs), external ventricular drains (EVDs), ventriculosubgaleal (VSG) shunts, or lumbar punctures (LPs) are treatment options in the management of PHH. Clinical judgment is required. Strength of Recommendation: Level II, moderate degree of clinical certainty.
Recommendation concerning Surgical temporizing measures: II. The evidence demonstrates that VSG shunts reduce the need for daily CSF aspiration compared with VADs. Strength of Recommendation: Level II, moderate degree of clinical certainty.
Recommendation concerning Routine use of Serial lumbar puncture: The routine use of serial lumbar puncture is not recommended to reduce the need for shunt placement or to avoid the progression of hydrocephalus in premature infants. Strength of Recommendation: Level I, high clinical certainty.
Recommendation concerning nonsurgical temporizing Agents: i. Intraventricular thrombolytic agents including tissue plasminogen activator (tPA), urokinase, or streptokinase are not recommended as methods to reduce the need for shunt placement in premature infants with PHH. Strength of Recommendation: Level I, high clinical certainty.
Recommendation concerning nonsurgical temporizing Agents. ii. Acetazolamide and furosemide are not recommended as methods to reduce the need for shunt placement in premature infants with PHH. Strength of Recommendation: Level I, high clinical certainty.
Recommendation concerning timing of Shunt placement: There is insufficient evidence to recommend a specific weight or CSF parameter to direct the timing of shunt placement in premature infants with PHH. Clinical judgment is required. Strength of Recommendation: Level III, unclear clinical certainty.
Recommendation concerning endoscopic third Ventriculostomy: There is insufficient evidence to recommend the use of endoscopic third ventriculostomy (ETV) in premature infants with posthemorrhagic hydrocephalus. Strength of Recommendation: Level III, unclear clinical certainty.
Recommendation: Neuro-endoscopic lavage is a feasible and safe option and therefore may be used for the removal of intraventricular clots and may lower the rate of shunt placement. Strength of Recommendation: Level III, unclear clinical certainty.
(http://thejns.org/doi/abs/10.3171/2014.7.PEDS14322)
Key Words: hydrocephalus, infant, case management, magnetic resonance imagining, posthemorrhagic hydrocephalus, premature infant, preterm infant, ventriculomegaly, intraventricular hemorrhage, meningitis, ventricular dilation, ventricular index, head circumference, in utero, shunt, reservoir, endoscopic third ventriculostomy, ventriculoperitoneal shunt, practice guidelines
Abbreviations used in this paper: AANS = American Association of Neurological Surgeons; CDC = Centers for Disease Control and Prevention; CNS = Congress of Neurological Surgeons; ELBW = extremely low birth weight; ETV = endoscopic third ventriculostomy; EVD = external ventricular drain; HUS = head ultrasound; IVH = intraventricular hemorrhage; LBW = low birth weight; LP = lumbar puncture; OFC = occipitofrontal circumference; PHH = posthemorrhagic hydrocephalus; PHVD = posthemorrhagic ventricular dilation; tPA = tissue plasminogen activator; VAD = ventricular access device; V/BP = ventricular/biparietal; VP = ventriculoperitoneal; VSG = ventriculosubgaleal.
Although reviews have been recently published, there exists a paucity of guidelines or evidence based recommendations for the management of posthemorrhagic hydrocephalus (PHH) in infants.1 According to 2007 data provided by the Division of Vital Statistics of the Centers for Disease Control and Prevention (CDC), infants born with very low birth weight and gestational age have a significantly higher risk of mortality.2 In fact, more than 50% of all infant deaths in 2007 occurred in infants born before 32 weeks’ gestation.2 In 2008, the reported preterm birth rate declined for the second consecutive year to 12.3%, but this decrease primarily involved those infants born in the later preterm period (34–36 weeks).3 Low birth weight (LBW) also contributes to increased infant mortality, and the CDC has reported that the percentage of LBW infants, or infants born weighing less than 2500 g, increased by 24% between 1984 and 2006.3
A recent study of 15,454 extremely low birth weight (ELBW) infants, each weighing between 401 g and 1000 g, was undertaken to assess neurodevelopmental outcome.4 More than 5000 infants died while in the hospital or before the follow-up visit. Among the 7693 children in whom follow-up studies were available, 2530 (33%) had a history of intraventricular hemorrhage (IVH). The IVH was Grade III or IV for 998 (13%) of the 7693 infants. Remarkably, in only 246 (3%) of the 7693 ELBW infants with follow-up was a shunt placed for PHH.4 There are still many questions about the optimal time to intervene for infants with PHH, and there are many different opinions about the best temporizing mechanism for symptomatic infants too small or unstable for permanent shunt placement.
The objective of this systematic review and analysis was to answer the following question: What are the optimal treatment strategies for posthemorrhagic hydrocephalus (PHH) in premature infants? We evaluated the current literature and constructed evidence-based recommendations supported by the strength of the available data for the management of PHH in premature infants. Specifically, we wanted to investigate relevant evidence for the following:
- Use of surgical temporizing methods such as ventricular reservoirs, external ventricular drains (EVDs), ventriculosubgaleal (VSG) shunts, and lumbar punctures (LPs).
- Routine use of serial LPs to reduce the need to shunt or to avoid the progression of hydrocephalus in premature infants.
- Use of intraventricular thrombolytic agents, including tissue plasminogen activator (tPA), urokinase, and streptokinase, to reduce the need for shunt placement in premature infants with PHH.
- Use of acetazolamide or furosemide to reduce the need for shunt placement in premature infants with PHH.
- Efficacy of endoscopic third ventriculosomy (ETV) in this population.
- Specific CSF parameters to direct the timing of shunt placement in premature infants with PHH.
Methods
Search Criteria
Both the US National Library of Medicine and the Cochrane Database of Systematic Reviews were queried using MeSH headings and key words relevant to PHH.
Key Words. The following key words were used in this study: (((preterm[All Fields] AND Intraventricular[All Fields] AND (“haemorrhage”[All Fields] OR “hemorrhage”[MeSH Terms] OR “hemorrhage”[All Fields])) OR ((“infant, premature”[MeSH Terms] OR (“infant”[All Fields] AND “premature”[All Fields]) OR “premature infant”[All Fields] OR (“preterm”[All Fields] AND “infant”[All Fields]) OR “preterm infant”[All Fields]) AND (“hydrocephalus”[MeSH Terms] OR “hydro cephalus”[All Fields]))) OR ((preterm[All Fields] AND (“heart ventricles”[MeSH Terms] OR (“heart”[All Fields] AND “ventricles”[All Fields]) OR “heart ventricles”[All Fields] OR “ventricular”[All Fields]) AND reservoir[All Fields])) AND shunt[All Fields].
Strategy
Two hundred thirteen abstracts were reviewed, after which 98 publications that met the inclusion criteria were selected. In addition to the overall inclusion/exclusion criteria specified in the Methods section of the Guidelines (Part 1), additional inclusion criteria included studies in which infants younger than 12 months with all forms of hydrocephalus—both congenital and acquired—were evaluated to ensure that the maximum number of studies were reviewed. The analysis focused on studies evaluating infants with PHH because of the treatment strategies and challenges unique to this patient population.
As a result of the US National Library of Medicine’s search engine functionalities, additional search terms (heart ventricles) not relevant to topics addressed in this chapter were added to the search strategy. Although these search terms remained in the search strategy, we did not recall any references retrieved using them for full-text review. We excluded those references because they were not relevant to the overall scope of this project or the patient population addressed in this chapter and, therefore, did not meet the article inclusion criteria specified in the methodology section of this guideline (Part 1).5
Following an evidentiary analysis and a review of the 98 full-text articles, 68 publications were accepted for inclusion in the evidentiary table and 30 publications were excluded.1,6-33 The evidentiary table was assembled linking recommendations to the strength of the evidence (Levels I–III).
Authors performed an updated literature search (in PubMed and Cochrane Central) for this guideline chapter through a medical librarian at the Congress of Neurological Surgeons Guidelines office using the below-mentioned existing search terms to update the original search through November 30, 2019.
Search Results
Fig. 1. Flowchart showing the process involved in identifying relevant literature. The criteria for “records excluded” and “fulltext articles excluded with reasons” are detailed in Part 1 of the Guidelines.
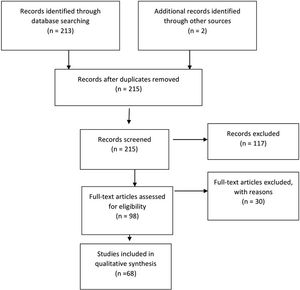
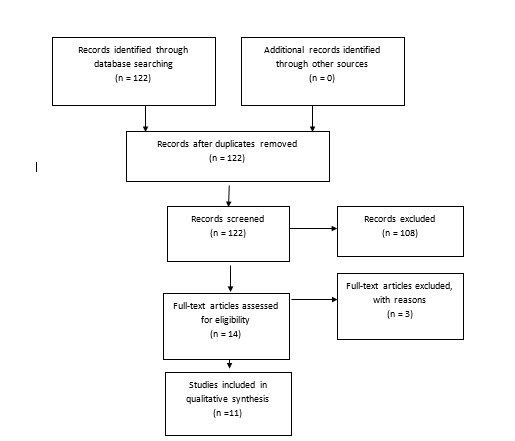
Fig. 2. Flowchart showing the process involved in identifying relevant literature for the 2020 Update. The criteria for “records excluded” and “full text articles excluded with reasons” are detailed in Part 1 of the Guidelines.
Of the 98 full-text articles selected for review, 30 full-text publications were rejected based on the criteria listed above and only 68 articles were used to construct the evidentiary table (Fig. 1). An additional 11 studies out of the 122 yielded by the 2020 update met inclusion criteria from the original guideline and were included (Figure 2). The criteria for the decision to treat were quite variable among different institutions and different study groups. For example, we evaluated 1 Class II study in which hydrocephalus was defined as the atrium of the lateral ventricle measuring > 10 mm on the horizontal plane of a head ultrasound (HUS) study or the body of the lateral ventricle at the level of the midthalamus measuring > 10 mm on a sagittal ultrasound image.34 We reviewed another Class III study in which hydrocephalus was defined as anterior cortical mantle thickness < 20 mm at an average postnatal age of 21 days along with increasing occipitofrontal circumference (OFC) as an indicator of hydrocephalus that should be treated.35 Bada et al35 reported that of 10 infants requiring shunts, 5 (50%) experienced normal development, which was defined by physical and neurological assessment and evaluation using the Denver developmental screening tool. Evan’s ratio, which is described as the lateral measurement of the ventricle across the frontal horns divided by the lateral measurement across the brain (biparietal diameter; also known as the ventricular/biparietal [V/BP] ratio) can also be used to describe the severity of PHH.36 The majority of studies that were evaluated based on an initial diagnosis of PHH on HUS, CT, and MRI studies were also used. Choudhury described mild hydrocephalus as a V/ BP ratio of 0.26–0.40, moderate hydrocephalus as a V/ BP ratio of 0.40–0.60, severe hydrocephalus as a V/BP ratio of 0.60–0.90, and extreme hydrocephalus as a V/ BP ratio of 0.91–1.0.36 These authors also reported that the thickness of the cortical mantle was not a statistically significant indicator of outcome because several infants with extreme hydrocephalus displayed normal motor development.36 One Class II and 1 Class III study indicated that when ventriculoperitoneal (VP) shunts were placed, even in cases of severe or extreme hydrocephalus, there were some infants with normal development and motor outcome (50 of 82 patients in the Choudhury study).35,36 Numerous studies have reported that good neurodevelopmental outcomes may be seen if and when infants with hydrocephalus are aggressively treated and cortical mantle thickness is restored.
Results
Surgical Temporizing Measures
Recommendation: Ventricular access devices (VADs), external ventricular drains (EVDs), ventriculosubgaleal (VSG) shunts, or lumbar punctures (LPs) are treatment options in the management of PHH. Clinical judgment is required. Strength of Recommendation: Level II, moderate degree of clinical certainty.
Recommendation: The evidence demonstrates that VSG shunts reduce the need for daily CSF aspiration compared with VADs. Strength of Recommendation: Level II, moderate degree of clinical certainty.
The evidence demonstrates that VADs reduce morbidity and mortality compared with EVDs. Three Class II and 7 Class III studies were included as evidence to support the first recommendation, and these lower-quality studies documented the safety and efficacy of VADs, or Ommaya reservoirs, for the aspiration of CSF, ventricular decompression, and lowering of intracranial pressure.37-46 The authors of 2 Class II studies reported that ventricular reservoirs may reduce the incidence of shunt infection as well as noninfectious shunt complications.38,41 In one Class II study and one class III study, repeated aspiration of CSF from a VAD did not significantly increase the risk of infection.41,44 Three Class III studies reported that ventricular reservoirs did not significantly reduce the need for permanent shunt placement.42,43,47 One Class III study reported that the use of VADs, compared with the use of continuous ventricular drainage, significantly reduced morbidity and mortality rates that were associated with the surgical treatment of PHH in LBW infants with reservoirs, instead of EVDs (Table 1).41
The placement of an EVD has also been used to treat hydrocephalus in preterm infants with PHH and is an option for these children, as shown in 1 Class II and 7 Class III studies.48-55 Three Class III studies reported that an EVD obviated the need for VP shunt placement in fewer than one-third of infants treated.48,52,54 More than 50% of preterm infants with PHH did require permanent VP shunt placement following removal of an EVD (95 out of 132 survivors required a shunt).48,52-55
It has been reported that placement of a VSG shunt may reduce the need for permanent shunt placement. The authors of Class II and Class III studies reported trends toward shunt independence, but the studies only enrolled 32 and 95 patients, respectively, and the results were not statistically significant.56,57 In their report of a Class II, retrospective historical cohort study, Lam and Heilman demonstrated that VSG shunting significantly reduced the need for daily CSF aspiration, which may decrease the risk of introducing a de novo CSF infection.56 A chi square test performed on their data indicated that a VSG shunt did significantly reduce the need for daily CSF aspiration when compared with a VAD (c2 = 19.2, df = 1, p = 0.000016, p < 0.05).56 This may reduce the risk of infection or other complications. A larger, prospective study reported a statistically significant decreased need for permanent CSF diversion in infants treated with VSG shunts.57 This study reported that 66% of infants (20 of 30) treated with VSG shunts required VP shunts and 33% (10 of 30) remained shunt free; this was compared with a group of infants treated with VADs in which 75% (49 of 65) required VP shunts and only 25% of infants (16 of 65) remained shunt free.57
In 2 studies, 1 intervention was compared to another with specific recommendations about the timing of the intervention for temporizing measures for the treatment of PHH in very LBW infants. In 1 Class III study, the authors compared early versus late intervention, as assessed by ventricular dilation in 5 collaborating neonatal centers.58 Ninety-five patients were subdivided into early intervention or late intervention groups, depending on their ventricular index at the time of initial treatment. Early treatment was safe and effective regardless of whether LP and/or reservoir placement was used. Early intervention was associated with a reduced requirement for a VP shunt (OR = 0.22) and reduced risk of moderate-to-severe disability.58 Additionally, there was a single Class III observational study of outcomes in which LPs, EVD, VSG shunts, and reservoirs were used.24 All interventional studies were found to be safe and effective.24
Routine Use of Serial Lumbar Puncture
Recommendation: The routine use of serial lumbar puncture (LP) is not recommended to reduce the need for shunt placement or to avoid the progression of hydrocephalus in premature infants. Strength of Recommendation: Level I, high degree of clinical certainty.
One Class I study was included, and it reported no statistical differences in outcomes of preterm infants with PHH treated with observation alone or infants treated with daily LP (Table 2).59 Lumbar puncture is often used early in the treatment of PHH, despite the fact that there is no statistically significant reduction in the need for a shunt or progression of PHH.59,60 In fact, LP neither predicts nor prevents the need for a permanent VP shunt.51 A second study, a Class III study, also reported no difference in adverse outcome regardless of whether infants were untreated or treated with serial LP.61 Without aggressive treatment of hydrocephalus and with persistent ventricular dilation, outcome was poor.61 Additionally, there was a single Class III study that concluded that repeated LPs may cause or contribute to subsequent shunt infection.62 Although LP may be useful for drawing off CSF as an immediate treatment for elevated intracranial pressure in infants with PHH, or for sampling CSF, we do not recommend the routine use of LP to eliminate the need for a VP shunt.61
Nonsurgical Temporizing Agents
Intraventricular Thrombolytic Agents. Recommendation: Intraventricular thrombolytic agents including tissue plasminogen activator (tPA), urokinase, or streptokinase are not recommended as methods to reduce the need for shunt placement in premature infants with PHH. Strength of Recommendation: Level I, high clinical certainty.
Based on 1 high-quality Class I study, the DRIFT procedure—Drainage, Irrigation, and Fibrinolytic Therapy (intraventricular tPA)—is not recommended for PHH.63 DRIFT did not significantly reduce shunt surgery or death, but it was associated with an increased rate of secondary IVH (Table 3).63 Forty-four percent (15 of 34) of infants in the DRIFT group died or required a shunt, compared with 50% (19 of 36) of infants who received standard treatment. Thirty-five percent (12 of 34) of preterm infants in the DRIFT study had secondary IVH, compared with 8% (3 of 34) who received standard treatment.63 These results differ from those of earlier Class II and Class III studies in which a decreased rate for the need for permanent shunt placement was reported when low-dose urokinase or fibrinolytic therapy with tPA was used for ventricular irrigation and clot reduction.33,64-66
Reviews conducted by Whitelaw and Odd63 have also revealed that intraventricular injection of streptokinase has not been shown to be beneficial.67 A single case report of intravenous streptokinase, published in 1998, suggested that there may be some benefit.68 This report was followed by an early Class III study that found benefit in a nonrandomized cohort of preterm infants with PHH who were treated with intravenous low-dose streptokinase.69 However, data from a later Class II study led to the conclusion that routine use of intraventricular streptokinase in PHH was not recommended.70 These studies were included in the 2007 Whitelaw and Odd Cochrane review,63,67 which argues against intravenous streptokinase for the treatment of PHH in preterm infants (Table 3).
Despite increased short-term morbidity and recurrent IVH, some benefits were noted in the DRIFT survivors.71 In the most recent Whitelaw study,71 the reduction in the primary long-term outcome—death or severe disability— at 2 years in the DRIFT group reached statistical significance when adjusted for sex, birth weight, and grade of IVH. Severe cognitive disability also was reduced, and this improvement in cognitive function was statistically significant. There was also a reduction in severe sensorimotor disability with DRIFT, but this clinical improvement did not reach statistical significance. The authors hypothesized that the greater effect on cognitive rather than sensorimotor function may be attributed to parenchymal infarction in the periventricular white matter, which was seen in about half of the infants enrolled in the trial.71
Acetazolamide and Furosemide.
Recommendation: Acetazolamide and furosemide are not recommended as methods to reduce the need for shunt placement in premature infants with PHH. Strength of Recommendation: Level I, high clinical certainty.
After our review of the literature, we found two Class I studies that reported that preterm infants with a diagnosis of PHH who were treated with acetazolamide and furosemide demonstrated higher risks of neurological complications, morbidity, and mortality (Table 4).72,73 The International Posthemorrhagic Ventricular Dilation (PHVD) Drug Trial Group reported that administration of acetazolamide plus furosemide leads to higher rates of shunt placement (relative risk 1.42) and morbidity (84% vs 60%) compared with standard therapy.72 Kennedy etal.73 reported that treatment of PHVD with acetazolamide and furosemide did not decrease the rate of shunt placement (64% in the acetazolamide/furosemide group vs 52% in the control group, not treated with acetazolamide/furosemide).73 However, treatment was associated with an increased rate of neurological morbidity (81% vs 66%).73
Treatment of PHVD with acetazolamide and furosemide was not recommended.73 One Class III study reported this treatment was not associated with VP shunt placement, but the severity of IVH (based on IVH grade) and the patient age at the time of IVH were significantly associated with the need for permanent CSF diversion.51 Kennedy et al. also noted that the ventricular index at time of entry into trial was the only factor significantly predictive of death or need for shunt, after multiple logistic regression analysis.73
Timing of Shunt Placement
Recommendation: There is insufficient evidence to recommend a specific infant weight or CSF parameter to direct the timing of shunt placement in premature infants with PHH. Strength of Recommendation: Level III, unclear degree of clinical certainty.
There were two Class III studies which evaluated the lower limits of infant weight at time of initial shunt insertion (Table 5).38,59 A weight of 1500 g was safely used as a criterion for VP shunt placement in the Benzel study.38 A single Class III study showed that CSF cell count, protein, and glucose levels were not statistically related to the occurrence of shunt failure or infection in the study population.74 The authors recommended that placement of the shunt be timed when the infant’s age, weight, and overall stability allow.74
Endoscopic Third Ventriculostomy
Recommendation: There is insufficient evidence to recommend the use of endoscopic third ventriculostomy (ETV) in premature infants with PHH. Strength of Recommendation: Level III, unclear degree of clinical certainty.
Although ETV was discussed in several full-text articles that we reviewed, there was insufficient evidence available for us to make a recommendation for or against its use for the treatment of PHH in premature infants (Table 6). Endoscopic third ventriculostomy for the treatment of hydrocephalus in infants and children will be discussed more thoroughly in subsequent chapters (in particular, Part 4).75
2020 Update
There was one new recommendation yielded by the update stating that that neuro-endoscopic lavage is a feasible and safe option for the removal of intraventricular clots and may lower the rate of shunt placement.76 The remaining new literature confirmed the previous recommendations.76-83 (Table 7)
There was no change in the Level I recommendation against the routine use of serial lumbar punctures (LP) to reduce the need to shunt or to avoid the progression of HC in premature infants. In the management of PHH, there is still insufficient evidence to recommend one surgical temporizing method over another. Authors suggested that ventriculo-subgaleal shunts (VSG) reduce the need of daily CSF aspiration as compared to other ventricular access devices (VAD) and that Ommaya type ventricular access reservoirs reduce in morbidity and mortality compared to external ventricular drains (EVD) (Level II). There were some benefits of VSG over VAD identified in 2014 and affirmed in 2019 (Level II).80-82 Intraventricular thrombolytic agents are still not recommended as a method to reduce the need for shunt placement in premature infants with PHH; there was no change in this Level I recommendation. Acetazolamide/ Furosemide are also not recommended as methods to reduce the need for shunt placement in premature infants with PHH (Level I).
Excluded Studies
We excluded 1 Class III study for low “preterm” patient representation (7 patients); in the review of 52 consecutive ETV procedures in 49 infants with hydrocephalus, most infants (31 patients) had aqueductal stenosis.84 Of the 7 infants with preterm PHH, 6 required a shunt even after ETV. Infants with PHH from premature birth did not benefit from ETV.84 We excluded another Class III study including patients with different etiologies for hydrocephalus.85 Although ETV was successful in 57% of patients (8 of 14), the majority of those infants had congenital aqueductal stenosis without PHH. In the remaining 6 patients, a VP shunt was needed. In 1 Class III single-institution retrospective case series, 18 preterm infants with PHH were treated initially with Ommaya reservoir placement: 1 patient died, 5 patients received a VP shunt, and 9 patients underwent ETV.86 Three patients did not require any further intervention. While overall, 59% were shunt free at the last follow-up, 5 of the 9 patients who were treated with ETV had to undergo repeated surgery for VP shunt placement. The authors recommended combining placement of an Ommaya reservoir with ETV to reduce shunt dependency for preterm infants with PHH.86 There was a large (101 patients) Class III multicenter, retrospective study evaluating the success rate of ETV in patients with hydrocephalus from subarachnoid hemorrhage, IVH, and/or CSF infection; a minority of the patients (25% [25 of 101]) had PHH of prematurity.87 Overall, ETV was successful in 52% of the infants with PHH of prematurity. Endoscopic third ventriculostomy was successful in 100% (13 of 13) of children with a history of preterm PHH, even though these patients were initially treated with a shunt. Endoscopic third ventriculostomy was unsuccessful in 12 of 12 infants treated with ETV as the first-line treatment, following preterm PHH. In patients with both hemorrhage and infection, ETV was not successful.87
Three studies were excluded from the update after full text review because (although the populations studied and results that were reported included some data about premature infants with PHH) there were mixed populations included and results for premature infants with PHH could not be separately identified, tracked or otherwise determined.88-90
Conclusions
Surgical Temporizing Measures
Recommendation: Ventricular access devices (VADs), external ventricular drains (EVDs), ventriculosubgaleal (VSG) shunts, or lumbar punctures (LPs) are treatment options in the management of posthemorrhagic hydrocephalus (PHH). Clinical judgment is required. Strength of Recommendation: Level II, moderate degree of clinical certainty.
Recommendation: The evidence demonstrates that VSG shunts reduce the need for daily CSF aspiration compared with VADs. Strength of Recommendation: Level II, moderate degree of clinical certainty.
The evidence demonstrates that VADs reduce morbidity and mortality compared with EVDs.
Routine Use of Serial Lumbar Punctures
Recommendation: The routine use of serial lumbar puncture (LP) is not recommended to reduce the need for shunt placement or to avoid the progression of hydrocephalus in premature infants. Strength of Recommendation: Level I, high clinical certainty.
Nonsurgical Temporizing Measures
Recommendation: Intraventricular thrombolytic agents including tissue plasminogen activator (tPA), urokinase, or streptokinase are not recommended as methods to reduce the need for shunt placement in premature infants with PHH. Strength of Recommendation: Level I, high clinical certainty.
Recommendation: Acetazolamide and furosemide are not recommended as methods to reduce the need for shunt placement in premature infants with PHH. Strength of Recommendation: Level I, high clinical certainty.
Timing of Shunt Placement
Recommendation: There is insufficient evidence to recommend a specific weight or CSF parameter to direct the timing of shunt placement in premature infants with PHH. Clinical judgment is required. Strength of Recommendation: Level III, unclear clinical certainty.
Endoscopic Third Ventriculostomy
Recommendation: There is insufficient evidence to recommend the use of endoscopic third ventriculostomy (ETV) in premature infants with PHH. Strength of Recommendation: Level III, unclear clinical certainty.
Recommendation: Neuro-endoscopic lavage is a feasible and safe option and therefore may be used for the removal of intraventricular clots and may lower the rate of shunt placement. Strength of Recommendation: Level III, unclear clinical certainty.
Acknowledgments
We acknowledge the American Association of Neurological Surgeons (AANS)/Congress of Neurological Surgeons (CNS) Joint Guidelines Committee for the members’ reviews, comments, and suggestions; the Hydrocephalus Association and Debby Buffa, patient advocate representative, for participation and input throughout the guidelines development; Pamela Shaw, research librarian, for her assistance with the literature searches; Kevin Boyer for his assistance with data analysis; and Sue Ann Kawecki for her assistance with editing.
We acknowledge the following individuals for their contributions throughout the review process: Timothy Ryken, M.D.; Kevin Cockroft, M.D.; Sepideh Amin-Hanjani, M.D.; Steven N. Kalkanis, M.D.; David P. Adelson, M.D.; Brian L. Hoh, M.D.; Mark D. Krieger, M.D.; Mark E. Linskey, M.D.; Jeffrey J. Olson, M.D.; Patricia Raskin, M.D.; Krystal L. Tomei, M.D.; and Monica Wehby, M.D. We also acknowledge the following peer reviewers for their contributions to review the update to the guidelines: Jennifer Sweet, MD, Brandon Rocque, MD, Christoph Greissenauer, MD, Jeffrey Olson, MD.
Disclosure
Dr. Limbrick receives research funding from the National Institute of Neurological Disorders and Stroke. The systematic review and evidence-based guidelines were funded exclusively by the CNS and AANS Pediatric Section, which received no funding from outside commercial sources to support the development of this document.
Conflict(s) of Interest: None. All Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Task Force members declared any potential conflicts of interest prior to beginning work on this systematic review and evidence-based guidelines.
Conflict(s) of Interest: None. All Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Update Task Force members declared any potential conflicts of interest prior to beginning work on this systematic review and evidence-based guidelines.
Author contributions to the study and manuscript preparation include the following. Conception and design: AANS/CNS Joint Section on Pediatrics. Acquisition of data: all authors. Analysis and interpretation of data: all authors. Drafting the article: Mazzola. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Flannery. Administrative/technical/material support: all authors. Study supervision: Flannery.
Table 1. Surgical Temporizing Measures Evidence Table
| First Author & Year |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
Cornips., et al 1997
|
Retrospective review of 14 patients with Grade III or Grade IV IVH diagnosed on HUS, treated with EVD. These 14 were compared to a historical cohort of 15 infants with similar Grade III/ IV IVH.
|
Class II
Retrospective review of 2 cohorts, those premature infants treated with EVD versus those treated medically. |
Ventricular drainage is a safe option for infants with PHH. |
|
Gurtner., et al 1992
|
Retrospective consecutively enrolled study of 736 low-birth-weight infants (< 1500 g). Twenty-seven infants were excluded because of incomplete records and 162 infants were not examined by cranial ultrasound because of early postnatal death, or if they were discharged before cranial ultrasound was done. The remaining 547 infants were reviewed in retrospective consecutive fashion. Criteria for shunt placement were clearly defined the presence of progressive HC documented by serial cranial ultrasounds, and OFC greater than the 95th percentile despite repeated lumbar punctures. All surgery was done in an operating room. All infants were followed for morbidity, mortality and infection. Data were examined for differences between the 3 years of the study, analyses of variance and Duncan's mean comparison tests were calculated. Chi-squared analyses were performed for yearly differences in discrete variables such as rates of complication and mortality. Student t test with Bonferroni corrections. Spearman correlation coefficients were computed when appropriate. Quantitative data were presented. |
Class II
Although this was a consecutive study of a high number of low birth weight infants, this was not a randomized, controlled study, but a non-random historical cohort, compared by year of treatment and treatment type. The temporizing method used in the first year was that of external ventricular drainage, whereas in subsequent years, a subcutaneous reservoir was used to intermittently drain the ventricles. Outcomes evaluated include: morbidity, mortality, and need for shunt revision.
|
Frequency and mortality of grades III and IV hemorrhage in infants weighing between 500 and 700 grams remained relatively constant over the 3 year period. Weights of these infants at time of surgery were rather low and did correlate with an increased rate of complication in 1988. However, That year, a high complication rate was associated with usage of external ventricular drains. The 3-month revision rate was 20% in 1987 and 50% in 1988; no shunts required revision in 1989. The group concluded that there was a significant reduction in morbidity and mortality associated with the surgical treatment of PHH in low-birth weight infants when they began using reservoirs, instead of external ventricular drains. |
|
Hudgins et al., 1997
|
Use of urokinase via reservoir to treat PHH in n=18 patients. Four different doses of urokinase; ultimately grouped into “high” (n=9) and “low” dose (everyone else, n=9). Both groups compared to historical control group with respect to outcome, need for shunt. Prospective, case control. |
Class II
Prospective, non-randomized, case-control series. Division of 9 patients into “low” dose group would appear to dilute statistical power, despite statistical significance obtained.
|
“Low dose” urokinase reduced shunt rate (71% vs 92%) compared to historical controls. Fewer shunt revisions in both groups compared to control group |
|
Lam et al., 2009
|
Single institution, retrospective historical cohort study of 32 preterm infants with PHH. This study compared 2 cohorts of infants: those treated with ventricular access device (VAD)/ Ommaya placement versus those treated with ventrciulosubgaleal shunts (VSG). There were no statistical differences in age or birth weight of the infants in the two groups. The groups were studied for IVH grade, need for daily CSF withdrawl, CSF leak from the scalp, CSF infection, and need for a VPS. |
Class II
A chi square
test was performed (X w =19.2, df=1, p value=0.000016, p<0.05) which showed that VSG significantly reduced the need of daily CSF aspiration as compared to VAD. The higher rate of complications
of VSG was not statistically significant to
the VAD group (p=0.17). 93.75% (15 of 16 patients) of the VAD group required VPS while 71.42% (10 of 14 patients) in the VSG group needed VPS.
|
There was a trend towards VPS independence in the VSG group, as compared to the VAD group, but it did not reach significance.
VSG did decrease the need for daily taps. There was a slightly higher rate of complications in the VSG group, but it was not significant.
|
|
Anwar et al., 1986
|
Consecutive, non-randomized study of 19 preterm infants with PHH who underwent placement of reservoirs for symptomatic HC. Symptomatic HC was defined as infants with rapidly increasing OFC, ventriculomegaly and signs of increased intracranial pressure were present, such as tense fontanelle, splayed sutures, apnea, bradycardia, seizure, feeding difficulties, or lethargy. |
Class III study was a case series study of the infants who were less than 200 grams, with clear CSF, and who were treated with reservoirs. There was only limited presentation of qualitative and quantitative data. Data were presented including: morbidity, mortality and need for shunt placement in these infants. There was no comparison to a cohort of non-treated infants or infants treated with ventricular drains.
|
The authors concluded that reservoirs provide safe and effective treatment for infants with PHH and symptomatic HC.
|
|
Benzel et al., 1993
|
41 patients requiring ventricular drainage for HC/ PHH were evaluated retrospectively. All drainage procedures were performed on patients with IVH with HC (Grade III [25 patients]) and IVH and IPH (Grade IV [16 patients]) who failed medical management. |
Class III
Retrospective case series of 41 consecutive premature infants. 26 ventricular reservoirs (Rickham or McComb reservoirs) were placed in neonates weighing less than 1500 grams, allowing for a safe but intermittent ventricular access. 18 of these reservoirs were subsequently converted to VPS. 32 % required a VPS and/or reservoir infection and 59% required a shunt revision during the first year of life.
No Grade IV patients achieved a normal functional level, while 10 Grade III patients did. The incidence of severe developmental delay (44% versus 28%) and death (38% versus 12%) was greater in the grade IV than the Grade III patients.
|
The placement of ventricular reservoirs is acceptable as an alternative to the early placement of ventriculo-peritoneal shunts. This approach may reduce the incidence of shunt infection as well as noninfectious shunt complications.
|
|
Berger et al., 2000
|
Retrospective review of outcomes after EVDs, n=37 preemies (n=51 drains), PHH diagnosed by ultrasound. |
Class III:
Single institution retrospective review
|
Neurodevelopmental outcome dependent on extent of parenchymal injury. Infection rate: 5.4%/patients, 3.9%drain. 11/37 did not require shunting.
|
|
Brouwer et al., 2007
|
Single center retrospective review of 76 preterm infants treated for PHVD with ventricular reservoirs. Infection rates were measured in two successive 6-year intervals. Number of reservoir punctures also examined. |
Class III
Single center retrospective review
|
While the number of reservoir punctures did not change, the infection rate was lower in the second, more recent interval (2/50 or 4% versus 5/26 or 19.2%). Conclusion: Risks associated with ventricular reservoirs are within acceptable limits.
|
|
de Vries et al., 2002
|
Retrospective review of consecutive preterm infants (EGA≤34 weeks) with Grade III IVH treated for post-hemorrhagic ventricular dilatation in 5 collaborating NICUs (n=95). Subjects were subdivided into early intervention or late intervention groups, depending on their ventricular index at the time of initial treatment. |
Class III
While this was a multi-center study, it was a retrospective case series. Treatments were not standardized (infants treated variably with LPs, reservoir, shunt) and neurodevelopmental outcome measures were limited.
|
Early treatment was associated with a reduced requirement for VP shunt (OR=0.22) and reduced risk of moderate-severe disability.
|
|
Gaskill et al., 1998
|
The use of a subcutaneous reservoir was studied in a consecutive, non-randomized series of 38 infants with preterm IVH and PHH. All infants had failed LP and medical treatment. |
Class III
This was a retrospective study of a series of premature infants who required temporizing measures (reservoir placement) after failing medical treatment/ LP for PHH. There were 28 survivors overall (8 died before a shunt, 2 died after a shunt). Four survivors (15%) did not require a shunt.
|
The authors concluded that early reservoir placement is feasible, safe and effective treatment of PHH associated with preterm IVH.
|
|
Harbaugh et al., 1981
|
Retrospective review of 11 premature infants with IVH and PHH were managed with tunneled EVD. The mean duration of drainage for this group was 20.7 days. No morbidity or mortality occurred as a result. 7/11 patients required a shunt. 2/11 have not required VPS. |
Class III
Small retrospective case review.
|
EVD via a subcutaneously tunneled catheter has been found to be a safe and reliable initial method of treating posthemorrhagic hydrocephalus in premature infants.
|
|
Heep et al., 2007
|
Safety/efficacy of Rickham reservoir placement for PHH patients. |
Class III
Retrospective review. Broad inclusion criteria for reservoir placement. No comparison to patients managed with other methods.
|
Ommaya / Rickham reservoir is a safe, effective option for managing PHH until ready for shunt. 5% infection rate, 85% of patients needed shunt.
|
|
Hudginsn et al., 1998
|
Use of VAD in n=149 PHH patients. Daily taps for first ‘several’ days (10-15 cm3/kg). |
Class III
Retrospective, case series from a single institution retrospective review. Shunts placed at 2kg if still symptomatic, but criteria not otherwise clear on when to stop VAD aspirations.
|
8% infection, 20% revision rates. 88% shunt implantation rate.
|
|
Kazan et al., 2005
|
Single-center, retrospective review of preterm and low birth weight infants diagnosed with intraventricular hemorrhage by ultrasound (n=42). 11 infants who required VP shunt were compared to 31 who did not. All subjects received acetazolamide and furosemide as an initial medical treatment. |
Class III
Small, retrospective case series with grouping of subjects despite variable treatments.
|
Risk factors for VP shunt included IVH grade, later EGA at birth, and age (days) at time of IVH, but not treatment for IVH/PHH (acetazolamide, furosemide, LP, and external ventricular drainage).
|
|
Kormanik et al., 2010
|
Kormanik et al. reported a retrospective review of the outcome of infants receiving a ventricular reservoir for PHH. |
Class III
Retrospective observational study, review of medical records of all infants receiving ventricular reservoir in one center between 2000 and 2007.
|
Ventricular reservoirs were placed in 35 infants during study period for management of hydrocephalus. Six (17%) were excluded. The remaining 29 infants had VR placement for PHVD. Serial tapping was performed on 681 occasions in 29 infants before placement of VP shunt or transport back to referring hospital. There were no cases of CSF culture-proven reservoir infection related to repeated taps, except for one case of Candida albicans CSF culture.
|
|
Kormanik et al., 2010
|
Kormanik et al. reported a retrospective review of the outcome of infants receiving a ventricular reservoir for PHH. |
Class III
Retrospective observational study, review of medical records of all infants receiving ventricular reservoir in one center between 2000 and 2007.
|
Ventricular reservoirs were placed in 35 infants during study period for management of hydrocephalus. Six (17%) were excluded. The remaining 29 infants had VR placement for PHVD. Serial tapping was performed on 681 occasions in 29 infants before placement of VP shunt or transport back to referring hospital. There were no cases of CSF culture-proven reservoir infection related to repeated taps, except for one case of Candida albicans CSF culture.
|
|
Limbrick et al., 2010
|
Large, single center retrospective review of 325 preterm infants with Grade 3 or 4 IVH. The development of PHH and the need for a temporizing device (VAD or VSG) were studied. Infections, complications and need for VPS were analyzed, as was mortality rate. |
Class III
Retrospective analysis showed 75.4% of the 65 infants treated with VAD needed a shunt; 66.7% of the 30 treated with VSG required a shunt. There was no significant difference in the infection rate between VAD and VSG, revision rate, or VPS infection afterwards,
|
There was no significant difference in outcome between infants treated with VAD or VSG.
|
|
Rahman et al., 1993
|
Single institution, small retrospective review of 37 patients with PHH, 31 of which required VPS. |
Class III
Observational study of outcomes; LP, EVD, VSG, and Ommaya reservoirs were used. No statistical data available.
|
Suggested LP, VSG, Ommaya and VPS are safe and effective.
|
|
Rhodes et al., 1987
|
Thirty-seven premature infants with PHH were treated with an EVD. Complications, including morbidity, were presented. 32 did not require a permanent shunt. Neurodevelopmental outcomes were presented along with neuromuscular outcomes. |
Class III
This was a retrospective, consecutive case series.
|
Level III Ventricular drainage is a safe and effective mechanism for treating infants with PHH and may obviate the need for a shunt.
|
|
Rhodes et al., 1987
|
Thirty-seven premature infants with PHH were treated with an EVD. Complications, including morbidity, were presented. 32 did not require a permanent shunt. Neurodevelopmental outcomes were presented along with neuromuscular outcomes. |
Class III
This was a retrospective, consecutive case series.
|
Level III Ventricular drainage is a safe and effective mechanism for treating infants with PHH and may obviate the need for a shunt.
|
|
Weninger et al., 1992
|
Study of 27 consecutive infants with an average gestational age of 31 weeks, with PHH and increased ICP who were treated with a tunneled EVD. PHH was defined as ventricular dilation, progressively increasing OFC, bulging fontanel, widening of the sutures, apnea or bradycardia. |
Class III
The study is a case series report.
|
PHH was successfully treated in all patients; the EVD was left in situ for an average of 23 +/- 9 days. 4 patients died of unrelated causes, and 23patients survived. 16 required shunts. Neurological outcome correlated with severity of the Grade of IVH. Grade 4 IVH infants had the worst neurological outcomes, despite treatment. The authors conclude that EVD is a safe and effective treatment for PHH in premature infants. |
|
Willis et al., 2009
|
Thirty-two premature infants with PHH treated with shunts were retrospectively reviewed to analyze the complications and outcome with respect to shunt revisions. Retrospective, consecutive case series of 32 infants who needed treatment for PHH. Multivariate analysis and time series were used to identify factors that influence the outcome in terms of shunt revisions. |
Class III
|
Reservoir placement suspended progression of hydrocephalus in only 2 patients, while permanent shunts were needed in 90.6% of cases. CSF reservoirs were a safe and effective method of treatment in infants considered too small for VP shunt placement but did NOT obviate the need for a shunt. |
|
Yu et al., 2009
|
The authors performed a retrospective case study of 11 premature infants with PHH all treated with a subcutaneous reservoir for CSF aspiration. |
Class III Retrospective case series.
|
The authors concluded that CSF reservoir treatment is safe and effective for infants with PHH. |
Table 2. Serial Lumbar Punctures Evidence Table
| Author |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
Anwar et al., 1986
|
Randomized controlled study of 47 consecutive pre-term infants with PHH with Grade 3 or Grade 4 IVH. Infants enrolled in the study were randomized to observation only or daily LP. Cohorts were studied for morbidity, mortality and need for a shunt.
|
Class I
Consecutively enrolled infants randomized to treatment (daily LP) (N= 24) or observation only (N=23). Ten infants treated with LP (10/24) required shunts and 9 of the observation-only group (9/23) required shunt placement for progressive PHH and HC. |
There were no statistical differences of outcomes studied in infants treated with observation alone or infants treated with daily LP. Although LP was safe, there was no statistically significant reduction in the need for shunt or progression of PHH. |
|
Behjati et al., 2011
|
Case series study that investigated risk factors for ventriculoperitoneal (VP) shunting in infants with HC following IVH in 97 consecutive pre-term infants with IVH. |
Class III
Case series of 97 infants with IVH associated with prematurity. The risks factors associated with the need for a shunt were investigated. Infants were followed for one year. Morbidities and mortalities were reported in a quantitative fashion. Patients treated medically with acetazolamide showed no benefit; however, infants treated with repeated CSF drainage through LP did have a higher shunt infection rate, once shunted.
|
Infants with Grade 3 or 4 IVH are at highest risk of PHH and HC and that the 11 of 31 patients who required a shunt developed shunt infection, which was significantly associated with repeat LP’s. |
|
|
Anwar et al., 1986
|
Randomized controlled study of 47 consecutive pre-term infants with PHH with Grade 3 or Grade 4 IVH. Infants enrolled in the study were randomized to observation only or daily LP. Cohorts were studied for morbidity, mortality and need for a shunt.
|
Class I
Consecutively enrolled infants randomized to treatment (daily LP) (N= 24) or observation only (N=23). Ten infants treated with LP (10/24) required shunts and 9 of the observation-only group (9/23) required shunt placement for progressive PHH and HC. |
There were no statistical differences of outcomes studied in infants treated with observation alone or infants treated with daily LP. Although LP was safe, there was no statistically significant reduction in the need for shunt or progression of PHH. |
|
|
Chaplin et al., 1980
|
Retrospective review of 22 consecutive, low birth weight infants with PHH. All developed HC after 2 weeks of age. The first 12 required VPS. In 10 infants born after September 1974, an attempt was first made to control the HC with repeated lumbar puncture and diuretics prior to placing a shunt. In 7/10 the hydrocephalus was successfully arrested by medical therapy alone. |
Class III
Retrospective review of 22 infants with PHH. There were two cohorts: 12 treated with VPS, and 10 treated with LP and diuretics. |
Follow-up at 1 to 8 years of age were done on 18 infants. 2/ 12 treated by permanent shunts and 3/6 treated medically had an IQ score of 85 or greater. These results indicate a poor long-term outlook for the low birth weight infant who develops clinically overt hydrocephalus after intracranial bleeding. |
|
Kazan et al. 2005
|
Single-center, retrospective review of preterm and low birth weight infants diagnosed with intraventricular hemorrhage by ultrasound (n=42). 11 infants who required VP shunt were compared to 31 who did not. All subjects received acetazolamide and furosemide as an initial medical treatment. |
Class III
Small, retrospective case series with grouping of subjects despite variable treatments. |
Risk factors for VP shunt included IVH grade, later EGA at birth, and age (days) at time of IVH, but not treatment for IVH/PHH (acetazolamide, furosemide, LP, external ventricular drainage). |
|
|
Muller et al., 1998
|
Effect of aggressive LP schedule on PHH. LPs started 0-4 days, on average 11LPs performed per patient, 15ml/kg or end of CSF flow per LP. Used protein, RBC count, glucose, ventricular size to determine endpoint. |
Class III
Non-randomized, prospective study, single institution. 16% complete resolution, 65% ventriculomegaly but not shunted, 19% shunted.. |
Serial LP should be started early for treatment of HC. |
|
|
Table 3. Intraventricular Thrombolytic Agents Evidence Table
| Author |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
Whitelaw et al., 2007
|
Randomized multicenter clinical trial examining standard treatment to DRIFT. 70 infants enrolled (34: DRIFT, 36: standard treatment). Outcomes at 6 months of age or hospital discharge: death or VP shunt surgery, secondary IVH, and infection.
|
Class I
Multicenter randomized controlled trial.
|
15/34 (44%) subjects in the DRIFT group expired or required a shunt, compared with 19/36 (50%) receiving standard treatment. 12/34 (35%) in DRIFT had secondary IVH, compared with 8% receiving standard treatment. Conclusion: DRIFT did not reduce shunt surgery or death but was associated with an increased rate of secondary IVH. |
|
Hudgins et al., 1997
|
Use of urokinase via reservoir to treat PHH in n=18 patients. 4 different doses of urokinase; ultimately grouped into “high ” (n=9) and “low” dose (everyone else, n=9). Both groups compared to historical control group with respect to outcome, need for shunt. Prospective, case control. |
Class II
Prospective, non-randomized, case-control series. Division of 9 patients into “low” dose group would appear to dilute statistical power, despite statistical significance obtained.
|
“Low dose” urokinase reduced shunt rate (71% vs 92%) compared to historical controls. Fewer shunt revisions in both groups compared to control group. |
|
|
Whitelaw et al., 2007
|
Review and meta-analysis of 2 prospective case-control studies (Luciano 1997, Yapicioglu 2003). Both source studies included 12 subjects total: 6 cases, 6 controls. meta-analysis.
|
Class II
Both sources studies were Class II (both were small randomized, prospective case-control studies).
|
No difference in mortality or VP shunt rate was observed with intraventricular streptokinase. Intraventricular fibrinolytic therapy cannot be recommended for infants following IVH. |
|
|
Yapicioglu et al., 2003
|
Single blind, prospective, study. Twelve preterm infants who developed posthaemorrhagic hydrocephalus were randomly assigned to the control group (no treatment) or to receive intraventricular streptokinase (x 3 days). Note: the streptokinase group also had an LP (10-15 cc) prior to treatment and then daily LPs (5-10cc). They also received intraventricular vancomycin. Primary outcome: VP shunt placement. |
Class II
Small randomized, prospective study
|
Five of 6 infants in the streptokinase group and 3 of 6 in the control group required VP shunts. No complications were noted. Routine use of intraventricular streptokinase in PHH was not recommended. |
|
Richard et al., 2001
|
Single institution experience with Ommaya reservoir in n=64 patients. N=17 patients received fibrinolytic therapy through Ommaya. |
Class III
Retrospective case series. Statistics performed on fibrinolytic therapy subgroup that consists of 2 different agents with multiple doses. Fibrinoytic subgroup then mixed back into overall outcome analysis.
|
Fibrinolytic therapy led to statistically significant lower rate of shunt (31% versus 87%). 22% infection rate. |
|
Whitelaw et al., 2003
|
Prospective Phase I trial of new treatment methodology (DRIFT) for prevention of PHH of prematurity. Data from 24 subjects compared with historical controls. Outcome measures: death, need for shunt, secondary IVH, infection, neurodevelopmental outcome. |
Class III
Prospective Phase I trial in 24 subjects and compared with historical controls.
|
One subject expired. 17 of 23 (74%) did not require a shunt. Two subjects experienced secondary IVH, and 2 experienced infections. 19 subjects >12 months had ND testing: 8 (42%) were normal, 7 (37%) had a single disability; 4 (21%) had multiple disabilities. Conclusion: Compared with historical controls, DRIFT reduced the need for shunts and showed a trend toward lower rates of mortality and disability. |
|
|
Whitelaw et al., 1992
|
Prospective study of 9 preterm infants with progressive post-hemorrhagic ventricular dilation who underwent a 48-72 hour continuous intraventricular infusion of streptokinase. Outcomes: death, need for shunt, secondary IVH, and infection. |
Class III
Small, prospective, non-randomized cohort study (Phase I trial).
|
All subjects survived; only 1 of 9 required a shunt prior to discharge (later reports indicated that a total of 4 of 9 ultimately required shunts). No infections, one re-hemorrhage. |
|
Whitelaw et al., 1996
|
Phase I study l to evaluate safety of tPA in 22 preterm infants with post-hemorrhagic ventricular dilation. Dose-finding data reported. Outcome measures: death and need for shunt prior to discharge and secondary IVH. |
Class III
Small, prospective, non-randomized cohort study (Phase I trial).
|
Dose-finding and pharmacokinetic data reported ([tPA], half-life tPA). 21 of 22 (95%) of subjects survived, 9/21 (45%) required shunts. One subject experienced secondary IVH. Conclusion: tPA resulted in survival without shunting in most subjects. |
|
|
Table 4. Acetazolamide/Furosemide Evidence Table
| Author |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
International PHVD Drug Trial Group, 1998
|
Use of ACTZ and furosemide in PHH patients. Comparison with standard therapy for shunt placement and neurologic outcome.
|
Class I
Randomized, controlled, multicenter, well-designed
|
ACTZ+furosemide leads to higher rates of shunt placement (RR 1.42) and higher morbidity (84% versus 60%) compared with standard therapy |
|
Kennedy et al., 2001
|
Multicenter, randomized, controlled trial designed to test the hypothesis that treatment of PHVD with acetazolamide and furosemide (vs standard therapy) would reduce: 1) risk of shunt placement or death before 1 year, 2) death or disability at 1 year. 177 subjects recruited from 55 centers worldwide. |
Class I
Multicenter RCT.
Positive: Excellent subject retention. Therapeutic CSF removal in 56% of subjects (equivalent in both groups). Negative: Acetazolamide and furosemide were stopped in many subjects due to adverse effects. Also, furosemide was given in the std therapy group in some cases.
|
Treatment of PHVD with acetazolamide and furosemide did not decrease the rate of shunt placement (64% in acetazol/furosemide group versus 52%; RR=1.23, CI=0.95-1.59) and was associated with increased neurological morbidity (81% vs 66%). Treatment of PHVD with acetazolamide and furosemide cannot be recommended. |
|
|
Kazan et al., 2005
|
Single-center, retrospective review of preterm and low birth weight infants diagnosed with intraventricular hemorrhage by ultrasound (n=42). Eleven infants who required VP shunt were compared to 31 who did not. All subjects received acetazolamide and furosemide as an initial medical treatment.
|
Class III
Small, retrospective case series with grouping of subjects despite variable treatments.
|
Risk factors for VP shunt included IVH grade, later EGA at birth, and age (days) at time of IVH, but not treatment for IVH/PHH (acetazolamide, furosemide, LP, external ventricular drainage). |
|
Table 5. Timing of Shunt Placement: Specific Weight or CSF Parameter Evidence Table
| Author |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
Anwar et al., 1986
|
Consecutive, non-randomized study of 19 preterm infants with PHH who underwent placement of reservoirs for symptomatic HC. Symptomatic HC was defined as infants with rapidly increasing OFC,
ventriculomegaly and signs of increased intracranial pressure were present, such as tense fontanelle, splayed sutures, apnea, bradycardia, seizure, feeding difficulties, or lethargy.
|
Class III
Case series study of the infants who were less than 200 grams, with clear CSF, and who were treated with reservoirs. There was only limited presentation of qualitative and quantitative data. Data were presented including: morbidity, mortality and need for shunt placement in these infants. There was no comparison to a cohort of non-treated infants or infants treated with ventricular drains.
|
The authors concluded that reservoirs provide safe and effective treatment for infants with PHH and symptomatic HC. |
|
Benzel et al., 1992
|
Forty-one patients requiring ventricular drainage for HC/ PHH were evaluated retrospectively. All drainage procedures were performed on patients with IVH with HC (Grade III [25 patients]) and IVH and IPH (Grade IV [16 patients]) who failed medical management. |
Class III: Retrospective case series of 41 consecutive premature infants. 26 ventricular reservoirs (Rickham or McComb reservoirs) were placed in neonates weighing less than 1500 grams, allowing for a safe but intermittent ventricular access. Eighteen of these reservoirs were subsequently converted to VPS. 32 % required a VPS and/or reservoir infection and 59% required a shunt revision during the first year of life. No grade IV patients achieved a normal functional level, while 10 grade III patients did. The incidence of severe developmental delay (44% versus 28%) and death (38% versus 12%) was greater in the grade IV than the grade III patients.
|
The placement of ventricular reservoirs is acceptable as an alternative to the early placement of ventriculo-peritoneal shunts. This approach may reduce the incidence of shunt infection as well as noninfectious shunt complications. |
|
|
Elgamal et al., 2011
|
Review of 52 consecutive ETV procedures for 49 infants with HC NOT necessarily associated with preterm IVH. Most infants (N=31) had aqueductal stenosis. The remainder of infants with HC had other causes of HC including Chiari II, Dandy-Walker cysts, quadrigeminal lipoma, CPA arachnoid cyst. Only 6 had PHH caused by preterm IVH.
|
Class III
Case series of infants treated with an ETV. Infants were followed for 68 months on average. Six of the seven infants with PHH from premature birth required a shunt.
|
The authors concluded that the success rate of 69.4% indicates that ETV is safe and effective in infants with HC not associated with PHH and prematurity. Infants with PHH from premature birth did not benefit from ETV. |
|
|
Fulkerson et al., 2011
|
Premature infants with PHH have a high risk of shunt obstruction and infection. Risk factors for complications include grade of IVH and age at shunt insertion. There is anecdotal evidence that the amount of red blood cells or protein levels in the CSF may also increase shunt complications. This study examined whether any relationship exists between the CSF constituents and shunt malfunction or infection.
|
Class III
This was a retrospective, cohort study evaluating the risk factors for shunt failure in preterm infants with IVH and PHH. Inclusion criteria and pre-intervention data points (baselines) were well documented. Outcomes reported were: early shunt failure or infection within 3 months of shunt. Statistical analysis was done using SAS version 9.2 for Windows (SAS Institute). Ordinary binary log regression was performed in which each CSF parameter was modeled as a possible predictor of the presence or absence of shunt malfunction or infection. Statistical significance was set at a probability level < 0.05.
|
The authors concluded that neither CSF cell count nor protein or glucose levels were statistically related to the occurrence of shunt failure or infection in the study population. The authors recommend that the placement of the shunt be timed when age, weight, and overall stability of the infant allow. |
|
Table 6. Endoscopic Third Ventriculostomy for PHH in Premature Infants Evidence Table
| Author |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
Elgamal et al., 2011
|
Review of 52 consecutive ETV procedures for 49 infants with HC NOT necessarily associated with preterm IVH. Most infants (N=31) had aqueductal stenosis. The remainder of infants with HC had other causes of HC including Chiari II, Dandy-Walker cysts, quadrigeminal lipoma, CPA arachnoid cyst. Only 6 had PHH caused by preterm IVH.
|
Class III
Case series of infants treated with an ETV. Infants were followed for 68 months on average. Six of the seven infants with PHH from premature birth required a shunt.
|
The authors concluded that the success rate of 69.4% indicates that ETV is safe and effective in infants with HC not associated with PHH and prematurity. Infants with PHH from premature birth did not benefit from ETV. |
|
Lipina et al., 2008
|
Retrospective, consecutive case series of 14 infants less than 6 month of age presenting with obstructive hydrocephalus. Eight of the 14 patients had post-hemorrhagic hydrocephalus. ETV was considered successful when VP shunt was not necessary. |
Class III
This study included a small number of patients with very different etiologies for hydrocephalus.
|
ETV was successful in 57%, the majority of them with primary aqueductal stenosis. In the remaining six patients, a VP shunt was needed. |
|
|
Peretta et al., 2007
|
Single institution retrospective review of 18 consecutive preterm infants with PHH. Subjects were treated with placement of an Ommaya reservoir for temporizing ventricular decompression. When necessary, subjects later underwent VP shunt placement (5) or ETV (9).
|
Class III
Small, single-institution retrospective case series with variable treatment patterns. Three of the surviving 17 infants (17.6%) treated with Ommayas did not require additional surgery. 14 of 17 required VP shunt (5) or ETV (9). While additional surgeries were required in the majority of cases, 59% were shunt-free at last follow-up.
|
Recommended combining Ommaya placement with ETV reduces shunt-dependency in this condition. |
|
|
Siomin et al., 2002
|
Multicenter, retrospective case series of 101 patients who had ETV for hemorrhage or infection. Both pediatric and adult subjects included. Of the 101 subjects, 25 were treated for PHH of prematurity, and specific data was reported for this cohort. Successful ETV defined as no further hydrocephalus operations required.
|
Class III multi-center study with a minority of subjects (25%) with PHH of prematurity.
|
ETV was successful in 52% of subjects with PHH of prematurity. Note that ETV was successful in 13/13 of PHH subjects previously treated with a shunt, whereas it was unsuccessful in 12/12 treated with ETV as the first-line treatment. ETV was not successful in subjects with both hemorrhage and infection. |
|
Table 7. New evidence included in 2020 Update
| Author |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
Bassan et al, 2012
|
32 premature infants with PHH retrospectively put into two groups: early (n ¼ 10) or late (n ¼ 22) EVD. The Battelle Developmental Inventory II and neuro-motor exam was then done at the median age of 73months.
|
Class III
|
In premature infants with PHH and Grade 1-3 IVH, early EVD placement was associated with lower rates of cognitive, communication and social disabilities.
|
|
Bock et al, 2018
|
Retrospective analysis; of premature infants w PHH. VAD or VPS. 72 patients. Time to first shunt revision and the mean number of shunt revisions through a span of 5 years after initial treatment were studied. Gestational age (GA), extent of IVH, and timing and placement of VAD or VPS were analyzed. |
Class III
|
Low GA and high grade IVH in preterm neonates with PHH with VPS show no significant impact on time to first shunt revision (i.e., revision-free shunt survival), but older GA infants had lower revision rates after 5 years of follow up. Temporizing measures may delay permanent VPS insertion and decrease revision rates through 5 years after VPS placement. |
|
Chamiraju et al, 2014
|
Seventeen (63%) of 27 infants w PHH required a VPS after ETV/CPC. Several factors studied were associated with a higher rate of failure: Grade IV hemorrhage,weight lessthan 3 kg, age younger than 3 months, need for reservoir placement, and presence of a normal cerebral aqueduct on MRI. Two factors were statistically significant: the patient’s corrected gestational age of less than 0 weeks at surgery and a narrow pre-pontine cistern on MRI. The majority (83%) of ETV/CPC failures occurred in the first 3 months after the procedure.
|
Class III
|
ETV/ CPC is feasible but failed in 63% of infants with PHH. |
|
Christian et al, 2016
|
Retrospective study of 91 preterm infants with PHH. 50 received VAD that was serially tapped. 41 received shunt placement. Of the infants with VAD, 5/50 (10%) did not undergo subsequent shunt placement. Number of shunt revisions and the rates of loculated HC and infection did not differ between the 2 groups.
|
Class III.
|
Infants who required a VAD had earlier surgery and 10% were able to avoid VPS. There were no differences in the number of shunt revisions, loculated hydrocephalus, and shunt infection among those infants who had VAD first, as compared to VPS as an initial treatment. |
|
De Vries et al, 2019
|
Multi-center RCT of 126 preterm infants ≤34 weeks gestation with VM after grade III–IV IVH. Infants randomized to low threshold (LT) (ventricular index (VI) >p97 and anterior horn width (AHW) >6 mm) or higher threshold (HT) (VI>p97+4 mm and AHW>10 mm).
|
Class III
|
NO significant difference in VPS placement or death in infants with PHH who were treated at a lower threshold. Infants treated at the lower threshold had more invasive procedures. Assessment of neurodevelopmental outcomes will provide information in defining the risks and benefits of both options. |
|
Riva-Cambrin et al, 2012
|
Use of temporizing devices and conversion to VPS was examined in 110 consecutive neonates surgically treated for IVH related to prematurity from the 4 clinical centers of the Hydrocephalus Clinical Research Network (HCRN). Clinical, imaging, and other care factors were analyzed. Seventy-three (66%) of the patients underwent temporization surgeries, including 50 VAD and 23 SGS. Center (p < 0.001), increasing ventricular size (p = 0.04), and bradycardia (p = 0.07) were associated with the use of a temporizing device, whereas apnea, occipitofrontal circumference (OFC), and fontanel were not. Implanted temporizing devices were converted to VPS in 65 (89%) of the 73 neonates. Only a full fontanel (p < 0.001) and VM (p =0.002) were associated with conversion to VPS.
|
Class III
|
Center variability exists in temporization of IVH in prematurity within the HCRN; however, variability between centers is not seen with VPS. VM—rather than clinical findings such as increasing OFCs—represents the threshold for either temporization or VPS. |
|
Schulz et al, 2014
|
Retrospective cohort study of twho groups of premature infants w PHH. 19 neonates treated w neuro-endoscopic lavage and removal of clot. 10 PHH neonates were treated conventionally, initially using temporary CSF diversion via lumbar punctures, VAD, or EVD. Complications and VPS rates were evaluated. Patient groups did not differ regarding gestational age and birth weight. In the endoscopic lavage group, no procedure-related complications were observed. After endoscopic lavage, 11 (58%) of 19 patients had VPS, as compared with 100% of infants treated conventionally (p < 0.05). Endoscopic lavage infants had fewer numbers of procedures (median 2 vs 3.5per patient, respectively; p =0.08), significantly fewer infections (2 vs 5 patients, respectively; p < 0.05), or multi-loculated HC (0 vs 4), respectively; p < 0.01).
|
Class III
|
Feasibility and safety of neuro-endoscopic lavage for the treatment of PHH in neonates are presented. The nominally improved results (decreased shunt, infection and loculation rates) deserve further study. |
|
Tian et all, 2012
|
Retrospective, single center study of 310 premature infants w IVH. Of these, 28 required VAD. There were no infections associated with VAD and a very low rate of complications (repositioning 7.4%) or replacement (3.75%).
|
Class III
|
VAD is very safe, with few complications.
|
|
Wang et al, 2014
|
Retrospective analysis of 90 infants with IVH and PHH treated with VAD (n = 44) or VSGS (n = 46). The mean GA and weight were lower for VSGS patients (30.1 ± 1.9 weeks, 1.12 ± 0.31 kg) than for reservoir patients (31.8 ± 2.9 weeks, 1.33 ± 0.37 kg; p = 0.002 and p = 0.004, respectively). VAD was predictive of more taps prior to VPS compared withVSGS placement (10 ± 8.7 taps vs 1.6 ± 1.7 taps, p < 0.001). VPS placement was more delayed in VSGS patients as compared to AVD infants
(80.8 ± 67.5 days vs 48.8 ± 26.4 days, p = 0.012), so that VSGS patients weighed more at time of VPS (3.31 ± 2.0 kg vs 2.42 ±
0.63 kg, p = 0.016). VAD infants had more infections than VSGS (n = 9 [20.5%] vs n = 5 [10.9%], p = 0.21).
|
Class III
|
VAD and VSGS infection rates were similar but VSGS patients were significantly older and had achieved greater weights at the time of VPS. VSGS requires less taps.The potential differences in long-term developmental and neurological outcomes between VSGS and reservoir placement warrant further study.
|
|
Wellons et al, 2017
|
Multicenter HCRN prospective cohort study of 145 premature infants w Grade 3 or 4 IVH and PHH. Treatment decisions were standardized regarding timing of initial surgical treatment, upfront VPS versus temporization procedure (VR or VSGS), and when to convert a VR or VSGS to a VPS. The primary outcome was the proportion of infants who underwent conversion to a VPS. The secondary outcomes of interest included infection and complication rates.
|
Class III
|
Using standardized decision rubrics, 28 infants never reached the threshold for treatment, 11 initially received VPS, 4 were treated with ETV and 102 underwent a temporization (36 with VSGSs and 66 with VRs). The 2 temporization cohorts were similar in terms of sex, race, IVH grade, head (orbitofrontal) circumference, and ventricular size at temporization. There were statistically significant differences noted between groups in gestational age, birth weight, and bilaterality of clot burden that were controlled for in post hoc analysis. By Kaplan-Meier analysis, the 180-day rates of conversion to permanent shunts were 63.5% for VSGS and 74.0% for VR (p = 0.36, log-rank test). The infection rate for VSGS was 14% (5/36) and for VR was 17% (11/66; p = 0.71). The overall compliance with standardized decision process was 90% for all surgeons. VAD/ VR or VSGS have similar VPS conversion rates.
|
|
Zuchelli et al, 2016
|
10 ELBW infants w PHH (5 cases < 700 g, range for all cases 550–1000 g) were treated with a PTT EVD that was implanted at bedside. AVG procedure time was 7 minutes, and there was no blood loss. EVD stayed in place for ave of 24 days (range 8–45 days). In all cases early control of HC was achieved. There was one CSF leak and one contamination. One patient died of sepsis. Once a patient reached 1 kg a VPS was implanted if needed.
|
Class III
|
The introduction of PTT EVD placement for the management of PHH is feasible and safe.
|
References
- Robinson S. Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. Journal of neurosurgery Pediatrics. 2012;9(3):242-258.
- Mathews TJ, MacDorman MF. Infant mortality statistics from the 2007 period linked birth/infant death data set. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2011;59(6):1-30.
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Osterman MJ. Births: final data for 2008. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2010;59(1):1, 3-71.
- Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121(5):e1167-1177.
- Flannery AM, Mitchell L. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 1: Introduction and methodology. 2014;14(Supplement_1):3.
- Albright ALH, S. J.; Taylor, F. H. Function of parietal and frontal shunts in childhood hydrocephalus. Journal of neurosurgery. 1988;69(88):883-886.
- Benzel EC, Reeves JD, Kesterson L, Hadden TA. Slit ventricle syndrome in children: clinical presentation and treatment. Acta neurochirurgica. 1992;117(1-2):7-14.
- Bruinsma N, Stobberingh EE, Herpers MJ, Vles JS, Weber BJ, Gavilanes DA. Subcutaneous ventricular catheter reservoir and ventriculoperitoneal drain-related infections in preterm infants and young children. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2000;6(4):202-206.
- Burstein J, Papile LA, Burstein R. Intraventricular hemorrhage and hydrocephalus in premature newborns: a prospective study with CT. AJR American journal of roentgenology. 1979;132(4):631-635.
- Chaparro MJ, Pritz MB, Yonemura KS. Broviac ventriculostomy for long-term external ventricular drainage. Pediatric neurosurgery. 1991;17(4):208-212.
- Felderhoff-Mueser U, Buhrer C, Groneck P, Obladen M, Bartmann P, Heep A. Soluble Fas (CD95/Apo-1), soluble Fas ligand, and activated caspase 3 in the cerebrospinal fluid of infants with posthemorrhagic and nonhemorrhagic hydrocephalus. Pediatr Res. 2003;54(5):659-664.
- Fernell E, Hagberg G, Hagberg B. Infantile hydrocephalus in preterm, low-birth-weight infants--a nationwide Swedish cohort study 1979-1988. Acta Paediatrica. 1993;82(1):45-48.
- Haines SJ, Lapointe M. Fibrinolytic agents in the management of posthemorrhagic hydrocephalus in preterm infants: the evidence. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1999;15(5):226-234.
- Horinek D, Cihar M, Tichy M. Current methods in the treatment of posthemorrhagic hydrocephalus in infants. Bratislavske lekarske listy. 2003;104(11):347-351.
- Inagaki T, Kawaguchi T, Yamahara T, et al. Management of intraventricular hemorrhage in preterm infants with low birth weight. Acta neurochirurgica Supplement. 2012;113:173-175.
- James HE. Spectrum of the syndrome of the isolated fourth ventricle in posthemorrhagic hydrocephalus of the premature infant. Pediatric neurosurgery. 1990;16(6):305-308.
- Korinth MC, Weinzierl MR, Gilsbach JM. Experience with a new concept to lower non-infectious complications in infants with programmable shunts. Eur J Pediatr Surg. 2003;13(2):81-86.
- Martinez-Lage JF, Almagro MJ, Del Rincon IS, et al. Management of neonatal hydrocephalus: feasibility of use and safety of two programmable (Sophy and Polaris) valves. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2008;24(5):549-556.
- McCullough DC. A critical evaluation of continuous intracranial pressure monitoring in pediatric hydrocephalus. Child's brain. 1980;6(5):225-241.
- Ment LR, Duncan CC, Ehrenkranz RA, et al. Randomized low-dose indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight neonates. The Journal of pediatrics. 1988;112(6):948-955.
- Miranda P. Intraventricular hemorrhage and posthemorrhagic hydrocephalus in the preterm infant. Minerva pediatrica. 2010;62(1):79-89.
- Morimoto K, Hayakawa T, Yoshimine T, Wakayama A, Kuroda R. Two-step procedure for early neonatal surgery of fetal hydrocephalus. Neurologia medico-chirurgica. 1993;33(3):158-165.
- Paraicz E. Successful treatment of perinatal intraventricular haemorrhage. Acta paediatrica Academiae Scientiarum Hungaricae. 1979;20(2-3):211-214.
- Perlman JM, Lynch B, Volpe JJ. Late hydrocephalus after arrest and resolution of neonatal post-hemorrhagic hydrocephalus. Developmental medicine and child neurology. 1990;32(8):725-729.
- Pople IK, Griffith HB. Control of hydrocephalus by endoscopic choroid plexus coagulation--long-term results and complications. Eur J Pediatr Surg. 1993;3 Suppl 1:17-18.
- Sasidharan P, Marquez E, Dizon E, Sridhar CV. Developmental outcome of infants with severe intracranial-intraventricular hemorrhage and hydrocephalus with and without ventriculoperitoneal shunt. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1986;2(3):149-152.
- Scavarda D, Bednarek N, Litre F, et al. Acquired aqueductal stenosis in preterm infants: an indication for neuroendoscopic third ventriculostomy. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2003;19(10-11):756-759.
- Tubbs RS, Banks JT, Soleau S, et al. Complications of ventriculosubgaleal shunts in infants and children. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2005;21(1):48-51.
- Tubbs RS, Smyth MD, Wellons JC, 3rd, Blount JP, Grabb PA, Oakes WJ. Alternative uses for the subgaleal shunt in pediatric neurosurgery. Pediatric neurosurgery. 2003;39(1):22-24.
- Warf BC, Campbell JW, Riddle E. Initial experience with combined endoscopic third ventriculostomy and choroid plexus cauterization for post-hemorrhagic hydrocephalus of prematurity: the importance of prepontine cistern status and the predictive value of FIESTA MRI imaging. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2011;27(7):1063-1071.
- Whitelaw A. Repeated lumbar or ventricular punctures in newborns with intraventricular hemorrhage. The Cochrane database of systematic reviews. 2001(1):CD000216.
- Whitelaw A, Kennedy CR, Brion LP. Diuretic therapy for newborn infants with posthemorrhagic ventricular dilatation. The Cochrane database of systematic reviews. 2001(2):CD002270.
- Whitelaw A, Pople I, Cherian S, Evans D, Thoresen M. Phase 1 trial of prevention of hydrocephalus after intraventricular hemorrhage in newborn infants by drainage, irrigation, and fibrinolytic therapy. Pediatrics. 2003;111(4 Pt 1):759-765.
- Boynton BR, Boynton CA, Merritt TA, Vaucher YE, James HE, Bejar RF. Ventriculoperitoneal shunts in low birth weight infants with intracranial hemorrhage: neurodevelopmental outcome. Neurosurgery. 1986;18(2):141-145.
- Bada HS, Salmon JH, Pearson DH. Early surgical intervention in posthemorrhagic hydrocephalus. Child's brain. 1979;5(2):109-115.
- Choudhury AR. Infantile hydrocephalus: management using CT assessment. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1995;11(4):220-226.
- Anwar M, Doyle AJ, Kadam S, Hiatt IM, Hegyi T. Management of posthemorrhagic hydrocephalus in the preterm infant. Journal of pediatric surgery. 1986;21(4):334-337.
- Benzel EC, Reeves JP, Nguyen PK, Hadden TA. The treatment of hydrocephalus in preterm infants with intraventricular haemorrhage. Acta neurochirurgica. 1993;122(3-4):200-203.
- Brouwer AJ, Groenendaal F, van den Hoogen A, et al. Incidence of infections of ventricular reservoirs in the treatment of post-haemorrhagic ventricular dilatation: a retrospective study (1992-2003). Archives of disease in childhood Fetal and neonatal edition. 2007;92(1):F41-43.
- Gaskill SJ, Marlin AE, Rivera S. The subcutaneous ventricular reservoir: an effective treatment for posthemorrhagic hydrocephalus. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1988;4(5):291-295.
- Gurtner P, Bass T, Gudeman SK, Penix JO, Philput CB, Schinco FP. Surgical management of posthemorrhagic hydrocephalus in 22 low-birth-weight infants. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1992;8(4):198-202.
- Heep A, Engelskirchen R, Holschneider A, Groneck P. Primary intervention for posthemorrhagic hydrocephalus in very low birthweight infants by ventriculostomy. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2001;17(1-2):47-51.
- Hudgins RJ, Boydston WR, Gilreath CL. Treatment of posthemorrhagic hydrocephalus in the preterm infant with a ventricular access device. Pediatric neurosurgery. 1998;29(6):309-313.
- Kormanik K, Praca J, Garton HJ, Sarkar S. Repeated tapping of ventricular reservoir in preterm infants with post-hemorrhagic ventricular dilatation does not increase the risk of reservoir infection. Journal of Perinatology. 2010;30(3):218-221.
- Willis B, Javalkar V, Vannemreddy P, et al. Ventricular reservoirs and ventriculoperitoneal shunts for premature infants with posthemorrhagic hydrocephalus: an institutional experience. Journal of neurosurgery Pediatrics. 2009;3(2):94-100.
- Yu B, Li S, Lin Z, Zhang N. Treatment of posthemorrhagic hydrocephalus in premature infants with subcutaneous reservoir drainage. Pediatric neurosurgery. 2009;45(2):119-125.
- !!! INVALID CITATION !!! {}.
- Berger A, Weninger M, Reinprecht A, Haschke N, Kohlhauser C, Pollak A. Long-term experience with subcutaneously tunneled external ventricular drainage in preterm infants. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2000;16(2):103-109; discussion 110.
- Cornips E, Van Calenbergh F, Plets C, Devlieger H, Casaer P. Use of external drainage for posthemorrhagic hydrocephalus in very low birth weight premature infants. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1997;13(7):369-374.
- Harbaugh RE, Saunders RL, Edwards WH. External ventricular drainage for control of posthemorrhagic hydrocephalus in premature infants. Journal of neurosurgery. 1981;55(5):766-770.
- Kazan S, Gura A, Ucar T, Korkmaz E, Ongun H, Akyuz M. Hydrocephalus after intraventricular hemorrhage in preterm and low-birth weight infants: analysis of associated risk factors for ventriculoperitoneal shunting. Surg Neurol. 2005;64 Suppl 2:S77-81; discussion S81.
- Kreusser KL, Tarby TJ, Taylor D, et al. Rapidly progressive posthemorrhagic hydrocephalus. Treatment with external ventricular drainage. Am J Dis Child. 1984;138(7):633-637.
- Rahman N, Murshid WR, Jamjoom ZA, Jamjoom A. Neurosurgical management of intraventricular haemorrhage in preterm infants. JPMA The Journal of the Pakistan Medical Association. 1993;43(10):195-200.
- Rhodes TT, Edwards WH, Saunders RL, et al. External ventricular drainage for initial treatment of neonatal posthemorrhagic hydrocephalus: surgical and neurodevelopmental outcome. Pediatric neuroscience. 1987;13(5):255-262.
- Weninger M, Salzer HR, Pollak A, et al. External ventricular drainage for treatment of rapidly progressive posthemorrhagic hydrocephalus. Neurosurgery. 1992;31(1):52-57; discussion 57-58.
- Lam HP, Heilman CB. Ventricular access device versus ventriculosubgaleal shunt in post hemorrhagic hydrocephalus associated with prematurity. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2009;22(11):1097-1101.
- Limbrick DD, Jr., Mathur A, Johnston JM, et al. Neurosurgical treatment of progressive posthemorrhagic ventricular dilation in preterm infants: a 10-year single-institution study. Journal of neurosurgery Pediatrics. 2010;6(3):224-230.
- Dykes FD, Dunbar B, Lazarra A, Ahmann PA. Posthemorrhagic hydrocephalus in high-risk preterm infants: natural history, management, and long-term outcome. The Journal of pediatrics. 1989;114(4 Pt 1):611-618.
- Anwar M, Kadam S, Hiatt IM, Hegyi T. Serial lumbar punctures in prevention of post-hemorrhagic hydrocephalus in preterm infants. The Journal of pediatrics. 1985;107(3):446-450.
- Muller W, Urlesberger B, Maurer U, et al. Serial lumbar tapping to prevent posthaemorrhagic hydrocephalus after intracranial haemorrhage in preterm infants. Wiener klinische Wochenschrift. 1998;110(18):631-634.
- Chaplin ER, Goldstein GW, Myerberg DZ, Hunt JV, Tooley WH. Posthemorrhagic hydrocephalus in the preterm infant. Pediatrics. 1980;65(5):901-909.
- Behjati S, Emami-Naeini P, Nejat F, El Khashab M. Incidence of hydrocephalus and the need to ventriculoperitoneal shunting in premature infants with intraventricular hemorrhage: risk factors and outcome. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2011;27(6):985-989.
- Whitelaw A, Evans D, Carter M, et al. Randomized clinical trial of prevention of hydrocephalus after intraventricular hemorrhage in preterm infants: brain-washing versus tapping fluid. Pediatrics. 2007;119(5):e1071-1078.
- Hudgins RJ, Boydston WR, Hudgins PA, Morris R, Adler SM, Gilreath CL. Intrathecal urokinase as a treatment for intraventricular hemorrhage in the preterm infant. Pediatric neurosurgery. 1997;26(6):281-287.
- Richard E, Cinalli G, Assis D, Pierre-Kahn A, Lacaze-Masmonteil T. Treatment of post-haemorrhage ventricular dilatation with an Ommaya's reservoir: management and outcome of 64 preterm infants. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2001;17(6):334-340.
- Whitelaw A, Saliba E, Fellman V, Mowinckel MC, Acolet D, Marlow N. Phase I study of intraventricular recombinant tissue plasminogen activator for treatment of posthaemorrhagic hydrocephalus. Archives of disease in childhood Fetal and neonatal edition. 1996;75(1):F20-26.
- Whitelaw A, Odd DE. Intraventricular streptokinase after intraventricular hemorrhage in newborn infants. The Cochrane database of systematic reviews. 2007(4):CD000498.
- Luciano R, Tortorolo L, Chiaretti A, Piastra M, Velardi F, Polidori G. Intraventricular streptokinase infusion in acute post-haemorrhagic hydrocephalus. Intensive care medicine. 1998;24(5):526-529.
- Whitelaw A, Rivers RP, Creighton L, Gaffney P. Low dose intraventricular fibrinolytic treatment to prevent posthaemorrhagic hydrocephalus. Arch Dis Child. 1992;67(1 Spec No):12-14.
- Yapicioglu H, Narli N, Satar M, Soyupak S, Altunbasak S. Intraventricular streptokinase for the treatment of posthaemorrhagic hydrocephalus of preterm. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2003;10(3):297-299.
- Whitelaw A, Jary S, Kmita G, et al. Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics. 2010;125(4):e852-858.
- International randomised controlled trial of acetazolamide and furosemide in posthaemorrhagic ventricular dilatation in infancy. International PHVD Drug Trial Group. Lancet (London, England). 1998;352(9126):433-440.
- Kennedy CR, Ayers S, Campbell MJ, Elbourne D, Hope P, Johnson A. Randomized, controlled trial of acetazolamide and furosemide in posthemorrhagic ventricular dilation in infancy: follow-up at 1 year. Pediatrics. 2001;108(3):597-607.
- Fulkerson DH, Sivaganesan A, Hill JD, et al. Progression of cerebrospinal fluid cell count and differential over a treatment course of shunt infection. Journal of neurosurgery Pediatrics. 2011;8(6):613-619.
- Limbrick DD, Jr., Baird LC, Klimo P, Jr., Riva-Cambrin J, Flannery AM. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 4: Cerebrospinal fluid shunt or endoscopic third ventriculostomy for the treatment of hydrocephalus in children. Journal of neurosurgery Pediatrics. 2014;14 Suppl 1:30-34.
- Schulz M, Buhrer C, Pohl-Schickinger A, Haberl H, Thomale UW. Neuroendoscopic lavage for the treatment of intraventricular hemorrhage and hydrocephalus in neonates. Journal of neurosurgery Pediatrics. 2014;13(6):626-635.
- Christian EA, Melamed EF, Peck E, Krieger MD, McComb JG. Surgical management of hydrocephalus secondary to intraventricular hemorrhage in the preterm infant. Journal of neurosurgery Pediatrics. 2016;17(3):278-284.
- de Vries LS, Groenendaal F, Liem KD, et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomised controlled trial. Archives of disease in childhood Fetal and neonatal edition. 2019;104(1):F70-f75.
- Riva-Cambrin J, Shannon CN, Holubkov R, et al. Center effect and other factors influencing temporization and shunting of cerebrospinal fluid in preterm infants with intraventricular hemorrhage. Journal of neurosurgery Pediatrics. 2012;9(5):473-481.
- Tian AG, Hintz SR, Cohen RS, Edwards MS. Ventricular access devices are safe and effective in the treatment of posthemorrhagic ventricular dilatation prior to shunt placement. Pediatric neurosurgery. 2012;48(1):13-20.
- Wang JY, Amin AG, Jallo GI, Ahn ES. Ventricular reservoir versus ventriculosubgaleal shunt for posthemorrhagic hydrocephalus in preterm infants: infection risks and ventriculoperitoneal shunt rate. Journal of neurosurgery Pediatrics. 2014;14(5):447-454.
- Wellons JC, 3rd, Shannon CN, Holubkov R, et al. Shunting outcomes in posthemorrhagic hydrocephalus: results of a Hydrocephalus Clinical Research Network prospective cohort study. Journal of neurosurgery Pediatrics. 2017;20(1):19-29.
- Zucchelli M, Lefosse M, Corvaglia L, et al. Introduction of percutaneous-tunneled transfontanellar external ventricular drainage in the management of hydrocephalus in extremely low-birth-weight infants. Journal of neurosurgery Pediatrics. 2016;18(1):1-6.
- Elgamal EA, El-Dawlatly AA, Murshid WR, El-Watidy SM, Jamjoom ZA. Endoscopic third ventriculostomy for hydrocephalus in children younger than 1 year of age. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2011;27(1):111-116.
- Lipina R, Reguli S, Dolezilova V, Kuncikova M, Podesvova H. Endoscopic third ventriculostomy for obstructive hydrocephalus in children younger than 6 months of age: is it a first-choice method? Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2008;24(9):1021-1027.
- Peretta P, Ragazzi P, Carlino CF, Gaglini P, Cinalli G. The role of Ommaya reservoir and endoscopic third ventriculostomy in the management of post-hemorrhagic hydrocephalus of prematurity. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2007;23(7):765-771.
- Siomin V, Cinalli G, Grotenhuis A, et al. Endoscopic third ventriculostomy in patients with cerebrospinal fluid infection and/or hemorrhage. Journal of neurosurgery. 2002;97(3):519-524.
- Bassan H, Eshel R, Golan I, et al. Timing of external ventricular drainage and neurodevelopmental outcome in preterm infants with posthemorrhagic hydrocephalus. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2012;16(6):662-670.
- Bock HC, Feldmann J, Ludwig HC. Early surgical management and long-term surgical outcome for intraventricular hemorrhage-related posthemorrhagic hydrocephalus in shunt-treated premature infants. Journal of neurosurgery Pediatrics. 2018;22(1):61-67.
- Chamiraju P, Bhatia S, Sandberg DI, Ragheb J. Endoscopic third ventriculostomy and choroid plexus cauterization in posthemorrhagic hydrocephalus of prematurity. Journal of neurosurgery Pediatrics. 2014;13(4):433-439.