Reposted with permission from ©AANS, 2014
J Neurosurg Pediatrics (Suppl) 14:35–43, 2014
AANS, 2014
(Original text of the guideline was edited to reflect the update. Please click here for the original publication.)
Pediatric hydrocephalus: systematic literature review and evidence-based guidelines
Part 5: Effect of valve type on cerebrospinal fluid shunt efficacy
UPDATE
Lissa C. Baird, MD,1 Catherine A. Mazzola, MD,2 Kurtis I Auguste, MD,3 Paul Klimo Jr.MD, MPH,4–6 Ann Marie Flannery, MD7
1Department of Neurological Surgery, Oregon Health & Science University, Portland, Oregon; 2Division of Pediatric Neurological Surgery, Goryeb Children’s Hospital, Morristown, New Jersey; 3Department of Neurosurgery, University of California, San Francisco, California; 4Semmes-Murphey Neurologic & Spine Institute; 5Department of Neurosurgery, University of Tennessee Health Science Center; and 6Le Bonheur Children’s Hospital, Memphis, Tennessee; and 7Department of Neurological Surgery, Saint Louis University, St. Louis, Missouri
Object. The objective of this systematic review was to examine the existing literature to compare differing shunt components used to treat hydrocephalus in children, find whether there is a superior shunt design for the treatment of pediatric hydrocephalus, and make evidence-based recommendations for the selection of shunt implants when placing shunts.
Methods. Both the US National Library of Medicine PubMed/MEDLINE database and the Cochrane Database of Systematic Reviews were queried using MeSH headings and key words chosen to identify publications comparing the use of shunt implant components. Abstracts of these publications were reviewed, after which studies meeting the inclusion criteria were selected. An evidentiary table was compiled summarizing the selected articles and quality of evidence. These data were then analyzed by the Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Task Force to consider evidence-based treatment recommendations.
Results. Two hundred sixty-nine articles were identified using the search parameters, and 43 articles were re- called for full-text review. Of these, 22 papers met the study criteria for a comparison of shunt components and were included in the evidentiary table. The included studies consisted of 1 Class I study, 11 Class II studies, and 10 Class III studies. The remaining 21 articles were excluded.
Conclusions. An analysis of the evidence did not demonstrate a clear advantage for any specific shunt component, mechanism, or valve design over another.
Recommendation: There is insufficient evidence to demonstrate an advantage for one shunt hardware design over another in the treatment of pediatric hydrocephalus. Current designs described in the evidentiary tables are all treatment options. Strength of Recommendation: Level I, high degree of clinical certainty.
Recommendation: There is insufficient evidence to recommend the use of a programmable valve versus a nonprogrammable valve. Programmable and nonprogrammable valves are both options for the treatment of pediatric hydrocephalus. Strength of Recommendation: Level II, moderate degree of clinical certainty. (http://thejns.org/doi/abs/10.3171/2014.7.PEDS14325)
Key Words: hydrocephalus, cerebrospinal fluid shunt, practice guidelines, programmable valve, antisiphon device
Abbreviations used in this paper: AANS = American Association of Neurological Surgeons; CNS = Congress of Neurological Surgeons.
Hydrocephalus is the most common condition treated by pediatric neurosurgeons. Successful management with cerebrospinal fluid shunt systems began after Nulsen and Spitz placed the first implantable shunt in 1949, using a stainless steel ball-valve system.1 Over the next 2 decades, shunt systems evolved to include distal slit valves, proximal slit valves, and diaphragm valves. The subsequent development of artificial valves and silicone tubing advanced shunt design dramatically. Simple differential pressure valves were initially engineered followed by a second generation of valves that included autoregulating, adjustable, antisiphon, and gravitational components.
The objective of this systematic review is to examine literature in which differing shunt components used to treat hydrocephalus in children are compared to find whether there is a superior shunt design for the treatment of pediatric hydrocephalus and to make evidence-based recommendations regarding the selection of shunt implants when placing shunts. Currently, many shunt system components are available to the pediatric neurosurgeon, and they function with a variety of pressure, flow, and siphon control characteristics. Shunt system design has evolved along with attempts to minimize failure rates. The initial use of simple differential pressure valves led to concerns about the disadvantages of siphoning and associated shunt obstruction, subdural hematoma, slit ventricle syndrome, overdrainage, and craniosynostosis. In an attempt to minimize these complications, antisiphon devices have been developed and integrated into shunt systems as intrinsic to the valve mechanism or as separate devices. The antisiphon device is designed to provide progressive resistance to flow to counteract the siphoning that occurs when negative pressure is exerted with vertical positioning. The later development of programmable valves allowed for purposeful alterations in valve function to be made without a surgical procedure.
The purpose of this evidence-based review is to critically evaluate available data on the efficacy of comparable shunt components to determine if one shunt component is superior to another. Additionally, we created evidence-based recommendations on the selection of shunt components based on the strength of the available data. Most of the available evidence focuses on the comparison of shunt valve designs. Study outcome variables accepted for the purposes of this review included shunt survival, shunt complications, development of slit ventricle syndrome, and development of signs or symptoms of overdrainage.
Methods
Search Criteria
The US National Library of Medicine (PubMed/ MEDLINE) and the Cochrane Database of Systematic Reviews were queried for the period January 1966 through March 2012 using MeSH headings and key words relevant to shunt system components as detailed below.
Search Terms
PubMed/MEDLINE
- (“Cerebrospinal Fluid Shunts”[MeSH]) “Hydro- cephalus”[MeSH:noexp]
- 1 AND (programmable OR nonprogrammable OR non-programmable OR siphon OR
antisiphon* OR anti- siphon* OR (“differential pressure” OR “fixed pressure”) OR valve*)
- Limit 2 to Child (0–18 years)
- Limit to English and Humans
Cochrane Database
- MeSH descriptor Child
- MeSH descriptor Infant
- 1 or 2 and (MeSH descriptor Cerebrospinal Fluid Shunts)
- 3 and (MeSH descriptor Hydrocephalus)
- (programmable OR nonprogrammable)
- 4 and 5
Search Results
The search yielded 269 abstracts, which were then reviewed for relevance to the demonstration of superiority of 1 shunt component over another. Forty-three articles were recalled for full-text review. Predetermined inclusion and exclusion criteria were used to review each of these articles in detail. Twenty-two articles were included in the final evidentiary table. Reasons for exclusion of full-text articles included the absence of a valid comparison group (n = 14),2-15 the absence of a valid outcome variable (n = 4),16-19 invalid study design (n = 2),20,21 and redundant patient population (n = 1) (Fig. 1).22
For each article included in the evidentiary table, the study type, summary findings, and major conclusions were recorded, and a preliminary data class was assigned. The Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Task Force met to discuss the ranking of the evidence and the classification of data. Recommendations were then made based on the strength of the data in the evidentiary table (Table 1). In these discussions, if disagreement was encountered among Task Force members, a blinded vote was held and a consensus or majority opinion was reached.
Authors performed an updated literature search (in PubMed and Cochrane Central) for this guideline chapter through a medical librarian at the Congress of Neurological Surgeons Guidelines office using the above-mentioned existing search terms to update the original search through November 30, 2019. (Fig. 2)
Results
The review process identified 1 Class I study, 11 Class II studies, and 10 Class III studies. Only one included article was rated as a Class I study, Kestle et al. (2000),23 in which the investigators performed a randomized controlled trial comparing 3 kinds of valves: all types of standard differential pressure valves, a Delta valve (Medtronic) with an antisiphon mechanism, and an Orbis-Sigma valve (Cordis) with a variable-resis- tance and flow-limiting mechanism. Three hundred forty- four patients were randomly assigned to a valve type and followed up until the time of first shunt failure. Assessed outcome variables included shunt obstruction, overdrain-age, ventricular loculations, and infection. The investigators did not find a significant difference in shunt survival between the 3 valve types in either the short-term (Drake 1998)22 or extended23 follow-up.
Eleven Class II studies24-34 in which differing valve types were compared also failed to demonstrate a superior valve when shunt survival was assessed. Jain et al26 (2000) conducted a prospective cohort study in which they compared shunts using a standard differential pressure valve with a Delta (Medtronic) flow- regulating valve. The authors found no significant difference in overall shunt survival (p = 0.72), with a 5-year survival rate of 58.6% for the differential pressure valves and 58.7% for the Delta valves. The authors did note a relative difference between the 2 groups in the incidence of overdrainage and infection. The differential pressure valve was associated with 4 cases of post-shunt subdural effusion or slit ventricle syndrome, while the Delta valve was associated with only 1 case of subdural effusion. The Delta valve group had 3 infections, whereas the differential pressure valve group had no infections. Warf et al34 (2005) conducted a prospective randomized trial in which they compared the Codman-Hakim microprecision valve with the more affordable Chhabra valve. Ninety children were evaluated after randomization for shunt malfunction, shunt migration, and wound complication. No significant differences in outcome variables were demon Part 5: Effect of valve type on CSF shunt efficacy strated between the 2 groups. Smely and Van Velthoven (1997) conducted a retrospective cohort review in which they compared 66 infants who underwent placement of a ventriculoperitoneal Cordis Orbis-Sigma valve system with 53 patients who underwent placement of a ventricu- loatrial Codman Holter Valve shunt system.41 Forty-eight percent of patients with the Orbis-Sigma valve required one or more revisions while 98.1% of patients with the Holter Valve required 1 or more revisions (p < 0.001). The difference in distal placement of the shunt system is a confounding factor when comparing valve types in this study.
The updated literature review yielded 71 new studies. After the abstracts review, 20 studies were selected for full text review, after which 13 studies were excluded.
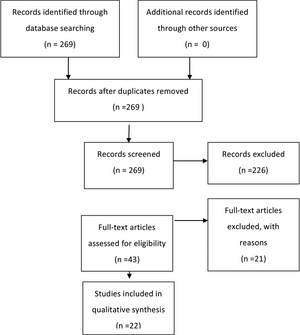
Fig. 1. Flowchart showing the process involved in identifying relevant literature.
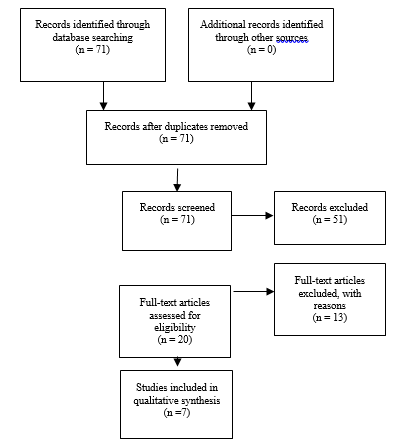
Fig. 2. Flowchart showing the process involved in identifying relevant literature for the 2020 Update. The criteria for “records excluded” and “full text articles excluded with reasons” are detailed in Part 1 of the Guidelines.
Antisiphon Mechanism
Three Class II studies evaluating the antisiphon mechanism were included in our review. Liniger et al28 (2003) studied 27 infants in a prospective cohort study in which a PS Medical medium pressure, flow-controlled valve was compared with a PS Medical 1.0 Delta valve with an antisiphon mechanism. The authors found a lower incidence of slit ventricle syndrome in the Delta valve group (6.25%) than in the flow-controlled valve group (9); however this finding did not reach statistical significance (p > 0.99). The incidence of shunt revision was also lower in the Delta group (0.12 revisions/patient/year) than in the flow-controlled valve group (0.19 revisions/ patient/year), a finding that also failed to reach statistical significance (p = 0.75). Khan et al27 (2010) studied the role of the antisiphon mechanism in a randomized con- trolled trial. Forty patients undergoing shunt placement were randomized to receive a differential pressure valve with an antisiphon device (Vygon shunt) or a differential valve without an antisiphon device (Chhabra or Ceredrain shunts). Shunt blockage, shunt infection, overdrainage, loculated ventricles, and occipitofrontal circumference were assessed in the 2 groups. No end point variables demonstrated a statistically significant difference. Over- drainage complications occurred in 10% of the patients in the group without an antisiphon device as opposed to 0% in the group with an antisiphon device (p = 0.48). A slightly higher infection and obstruction rate was noted in the antisiphon group. In a retrospective cohort study of 475 patients, Davis et al.4 (2000) assessed shunt survival and the development of subdural collection in patients treated with a Delta valve shunt with antisiphon function and in patients treated with one of 2 differential pressure valves without antisiphon control. In a comparison of the 3 groups, no significant difference was found.
The Class III studies that assessed the antisiphon mechanism include a retrospective review by Gruber et al35(1984), in which the authors evaluated 41 patients before and after primary or secondary placement of an antisiphon device. In the secondary placement group fewer complications and proximal catheter obstructions were noted after placement of such a device. However, no statistical analysis was provided by the authors to demonstrate the significance of their findings. In a retrospective cohort review of 101 patients who underwent shunt placement, Virella et al36 (2002) reported no significant differences between patients who underwent placement of a distal slit valve and patients who underwent placement of a Delta valve with an antisiphon component. The authors assessed the number of revisions, infections, and evidence of overdrainage, and reported that 31% of patients in the distal slit valve group required a single shunt revision and 8% required a second revision, whereas 30% of patients in the Delta valve group required a single revision and 20% required a second. Kaiser et al37(1997) reported a prospective but incompletely described comparison study between a conventional medium pressure valve and the Delta valve. The authors found no difference in the number of shunt revisions.
Slit Ventricles
Kan et al38(2007) conducted a retrospective review of 244 patients with at least 1 year of follow-up after primary shunt placement with a differential pressure valve, a Delta valve, or an Orbis-Sigma valve. Variables associated with the development of slitlike ventricles included patient age (younger age at insertion was associated with a higher incidence of slitlike ventricles; p = 0.09), etiology (trauma, infection, and aqueductal stenosis were associated with a higher incidence of slitlike ventricles), and valve type (10.8% of patients with differential pressure valves, 10.5% with Delta valves, and 3.6% with Orbis-Sigma valves developed slitlike ventricles; p = 0.007). This article suggests that a slower reduction in ventricle size and slower flow may lead to larger ventricles after shunt placement. Slit ventricle syndrome was not directly assessed; rather, the radiographic appearance of slitlike ventricles was used as a surrogate outcome.
Programmable Valves
Five Class II studies25,29-32 evaluated programmable valves. Pollack et al32 (1999) conducted a multicenter randomized controlled trial in which they compared the programmable Codman Hakim valve to the surgeon’s choice of any conventional valve. The authors demonstrated comparable efficacy and safety with no statistically significant difference in shunt survival between the experimental and control groups.
Hatlen et al. (2012) published an analysis of programmable and nonprogrammable valve survival.25 The programmable Strata and Codman Hakim valves were com- pared with multiple nonprogrammable valves and found to have significantly lower survival rates (19.8% vs. 45.8%, p = 0.0001). Another retrospective comparison between programmable valves (Strata or Codman-Medos) and non-programmable valves (Medtronic PS Medical) was under- taken by Mangano et al29 (2005). In that study 11% of the programmable valves malfunctioned compared with 0% of the nonprogrammable valves. The authors demonstrated a trend toward longer valve survival and shunt survival in the nonprogrammable group; however, neither reached statistical significance. McGirt et al. (2007) retrospectively reviewed 279 patients who had undergone shunt placement procedures involving either a programmable (Strata or Codman Hakim) or nonprogrammable (PS Medical Delta) valve.30 The authors found that programmable valve placement was associated with a reduced risk of both over- all shunt revision (35% vs 54% in the nonprogrammable group; p = 0.016) and proximal shunt obstruction (12% vs 28% in the nonprogrammable group; p = 0.006). Notarianni et al31 (2009) found no significant difference in a retrospective review of 253 patients who underwent shunt placement with either a programmable (Strata or Codman Hakim) or nonprogrammable (pressure-controlled or not specified) valve. The failure rate among the programmable valve group was 76.1%, and that among the differential pressure valve group was 80.0% (p = 0.11).
Other Comparison Groups
Several Class III studies comparing variable shunt valves were included in the review. Miranda et al39 (2011) describe a retrospective review of 103 patients who received shunts for preterm-related posthemorrhagic hydrocephalus. The authors reported a significantly higher rate of obstruction in patients weighing more than 2000 g who were treated with a fixed medium pressure valve (6 of 8 patients) than in those who were treated with a fixed low pressure valve (12 of 39 patients) (p = 0.040). Contrary findings were reported by Robinson et al. (2002) in a retrospective analysis of shunt malfunction variables in 158 patients.40 Valve opening pressure was the only significant controllable factor found to be associated with shunt malfunction. The 5-year shunt failure rate was 72% in the no valve or low pressure valve group and 47% in the medium or high pressure valve group (p = 0.0005).
Sainte-Rose et al41 (1991) reviewed the charts of 1719 patients with shunted hydrocephalus to assess mechanical complications. These authors found that the flanged ventricular catheter was associated with a higher risk of proximal occlusion (p < 0.04), open-ended distal catheters were associated with fewer distal obstructions (log-rank p < 0.0003), and shunts with proximal medium pressure valves were less likely to malfunction than shunts with distal slit valves (p < 0.000002). Tuli et al42 (2000) did not find valve type to be associated with shunt malfunction in a post hoc analysis of a prospective cohort of 839 patients who underwent primary shunt insertions. No association between shunt malfunction and any component of the shunt hardware was reported in that study.
Ramadwar et al43 (1997) retrospectively compared the efficacy of the Delta valve with the Heyer-Shulte Multi-Purpose valve in 28 patients. Sixty-nine percent of patients with the Delta valve required revision, compared with 53% of patients with the Multi-Purpose valve. The sample size in that study was small, and the data did not reach statistical significance. In an older paper by Serlo et al44 (1986), a retrospective review of 148 children was conducted to compare the Pudenz-Heyer valve with the Cordis Hakim valve. No significant difference was found in overall shunt efficacy, although significance was demonstrated in a higher rate of valve patency on the part of the Pudenz-Heyer valve (p < 0.001).
2020 update
Two studies supported the prior recommendations, including a large prospective cohort study by Riva-Cambrin et al45 which determined valve type had no impact on shunt survival and a retrospective comparison study between the paediGav and Codman Hakim valves by Beez et al46 which showed that valve type did not influence risk of shunt failure. Three studies25,47,48 were published demonstrating significant impact of valve type on shunt survival, however all three studies were class III data and therefore do not affect the original recommendations. Two class III studies49,50 were published demonstrating significance for unique outcome variables.
Excluded Studies
The Task Force excluded 21 articles recalled for full-text review from the final evidentiary table. The majority of excluded papers did not include a comparison group or control group.2-15 Other reasons for exclusion included invalid study design (questionnaire survey),20,21 redundant patient population22 (only the paper with the longest reported follow-up was included), and absence of a valid outcome variable (change in ventricle size, development of spinal canal stenosis, historical description, and frequency of hospital visits).16-19
Conclusions
Recommendation: There is insufficient evidence to demonstrate an advantage of one shunt hardware design over another for the treatment of pediatric hydrocephalus. Current designs described in the evidentiary tables are all treatment options. Strength of Recommendation: Level I, high degree of clinical certainty.
Recommendation: There is insufficient evidence to recommend the use of a programmable valve versus a nonprogrammable valve. Programmable and nonprogrammable valves are both options for the treatment of pediatric hydrocephalus. Strength of Recommendation: Level II, moderate degree of clinical certainty.
The available literature in which one shunt component is compared with another does not demonstrate a clear superiority of one over another. Higher rates of overdrainage were seen with standard differential pressure valves; however, the outcome variables studied in the comparisons of these groups with other shunt mechanisms failed to demonstrate statistical significance. While valves with antisiphon mechanisms may be superior in preventing overdrainage complications, no statistically significant data exist in the current medical literature to support this trend.
The studies assessing programmable versus nonprogrammable valves demonstrated either no statistically significant differences or contrary outcomes, pointing to the need for long-term prospective controlled analysis of this issue. Class III data demonstrating poorer function of distal slit valves in comparison with a proximal valve are described and are consistent with the contemporary decrease in utilization of the former type of shunt system. Many contemporary valve designs exist despite major deficiencies in long-term clinical evaluation. Well-de-signed comparison studies with clearly defined outcome variables and appropriate stratification of patient variables are needed to further investigate the appropriate clinical utilization of these valves. Accessing the necessary patient volume required to reach significance and balancing industry interests with trial integrity may be significant barriers to pursuing needed studies; however, as increasingly expensive and complex valves become available for clinical use, these studies will become imperative.
Based on the 2020 update, there is no new evidence to support a change in the original guideline recommendations.
Acknowledgments
We acknowledge the American Association of Neurological Surgeons (AANS)/Congress of Neurological Surgeons (CNS) Joint Guidelines Committee for the members’ reviews, comments, and suggestions; Laura Mitchell, Guidelines Project Manager for the CNS, for her contributions; Pamela Shaw, research librarian, for her assistance with the literature searches; Kevin Boyer for his assistance with data analysis; and Sue Ann Kawecki for her assistance with editing.
We also acknowledge the following peer reviewers for their contributions to review the update to the guidelines: Jennifer Sweet, MD, Brandon Rocque, MD, Christoph Greissenauer, MD, Jeffrey Olson, MD.
Disclosure
The systematic review and evidence-based guidelines were funded exclusively by the CNS and AANS Pediatric Section, which received no funding from outside commercial sources to support the development of this document.
Conflict(s) of Interest: None. All Task Force members declared any potential conflicts of interest prior to beginning work on this evidence review.
Conflict(s) of Interest: None. All Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Update Task Force members declared any potential conflicts of interest prior to beginning work on this systematic review and evidence-based guidelines.
Author contributions to the study and manuscript preparation include the following. Conception and design: AANS/CNS Joint Section on Pediatrics. Acquisition of data: all authors. Analysis and interpretation of data: all authors. Drafting the article: Baird. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Flannery. Administrative/technical/material support: all authors. Study supervision: Flannery.
Table 1. Valve Type Evidence Table
| Author |
Title |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
Kestle et al., 2000
|
Long-Term Follow-Up Data from the Shunt Design Trial
|
Multicenter randomized trial comparing differential pressure valve, Delta valve, and a Sigma valve. 344 patients with time to shunt failure as endpoint.
|
Class I: Randomized controlled trial
|
No clear advantage of one valve over another. |
|
Khan et al., 2010
|
Role of Shunts with Antisiphon Device in Treatment of Pediatric Hydrocephalus
|
Role of antisiphon device. 40 patients randomly assigned to shunt with ASD or differential valve.
|
Class II: Prospective, randomized. Small study with short follow-up (<6 months).
|
No overdrainage in antisiphon group, 2 patients with overdrainage in non-antisiphon group; higher occlusion and infection in ASD group. No endpoint variables reached statistical significance. |
|
Jain H, et al., 1999
|
The treatment of infantile hydrocephalus: "differential-pressure" or "flow-control" valves. A pilot study
|
Prospective data from 50 consecutive first-time shunt insertions. Comparison of shunt survival between differential pressure and flow-regulating (Delta) valves.
|
Class II: prospective cohort study
|
No significant difference in shunt survival between two groups: 5-year survival = 58.6% in DPV group and 58.7% in Delta valve group. Higher incidence of overdrainage in DPV group and higher rate of infection in Delta valve group though neither statistically significant. |
|
Kestle et al., 2000
|
Long-Term Follow-Up Data from the Shunt Design Trial
|
Multicenter randomized trial comparing differential pressure valve, Delta valve, and a Sigma valve. 344 patients with time to shunt failure as endpoint.
|
Class I: Randomized controlled trial
|
No clear advantage of one valve over another. |
|
Liniger et al., 2003
|
Flow Control versus Antisiphon Valves: Late Results Concerning Slit-Ventricles and Slit-Ventricle Syndrome
|
27 infants with flow-control vs antisiphon valves followed for development of slit ventricles and slit ventricle syndrome
|
Class II: prospective cohort study
|
No significant difference in development of slit ventricles or slit ventricle syndrome between 2 groups. Slit ventricle syndrome developed in 6.25% of antisiphon group and 9% of flow-controlled valve group. p>0.99 |
|
Pollack et al., 1999
|
A Randomized, Controlled Study of a Programmable Shunt Valve ersus a Conventional Valve for Patients with Hydrocephalus
|
377 patients randomized to receive the Codman Hakim programmable shunt versus a conventional valve of surgeon's choice and followed for valve explant and shunt failure.
|
Class II: multicenter randomized controlled trial. Control group not uniform in valve type.
|
Comparable efficacy and safety with no statistically significant difference in shunt survival. |
|
Davis at al., 2000
|
The Delta Valve: How Does its Clinical Performance Compare with Two other Pressure Differential Valves without Antisiphon Control
|
Retrospective cohort study of 475 patients undergoing VP shunt placement with Delta valve with antisiphon function or 2 differential pressure valves without antisiphon (Holter-Hausner and Heyer-Schulte). Endpoints included shunt survival and symptomatic subdural fluid collections
|
Class II: single institution retrospective cohort study
|
No significant difference in shunt survival at two-year follow-up: 65% Delta group 66% Holter-Hasner group 64% Heyer-Schulte valve group. No significant difference in rate of subdural fluid collection between groups. |
|
Hatlen et a., 2012
|
Nonprogrammable and programmable cerebrospinal fluid shunt valves: a 5-year study
|
Retrospective review of 523 patients undergoing 616 shunt surgeries with 2-year minimum follow-up. Patients with programmable valve placement (Strata and Codman Hakim valves) compared with non-programmable valves (Heyer Schulte, Spetz.er, Delta, and Medtronic). Valve survival was primary endpoint.
|
Class II: retrospective cohort study. Data obtained from prospectively collected shunt database
|
5-year survival for non-programmable valves (45.8%) was significantly higher than that for programmable valves (19.8%). p=0.0001 |
|
McGirt et al., 2007
|
Adjustable vs set-pressure valves decrease the risk of proximal shunt obstruction in the treatment of pediatric hydrocephalus
|
279 patients undergoing shunting procedures with either a programmable (Strata or Codman-Hakim) or a non-programmable valve (PS Medical Delta) were retrospectively reviewed and analyzed for time to shunt malfunction and type of malfunction.
|
Class II: retrospective cohort review
|
Programmable valves associated with reduced risk of both overall shunt revision (35% vs 54%, p=0.016) and proximal obstruction (12% vs 28%, p=0.006). |
|
Notarianni et al., 2009
|
Congenital hydrocephalus and ventriculoperitoneal shunts: influence of etiology and programmable shunts on revisions
|
253 patients undergoing shunting procedures with either a programmable (Medtronic Strata or Codman-Hakim) or a non-programmable (pressure-controlled or not specified) valve were retrospectively reviewed and analyzed for time to shunt malfunction.
|
Class I: Randomized controlled trial
|
Failure rates (p=0.11) were not significantly different between shunts with programmable valve (76.1%), shunts with non-programmable valve (80.0%), and shunts with non-specified valve (65.0%). |
|
Warf B 2005
|
Comparison of 1-year outcomes for the Chhabra and Codman-Hakim Micro Precision shunt systems in Uganda: a prospective study in 195 children
|
90 patients randomized to receive the Chhabra or Codman-Hakim shunt as primary treatment for hydrocephalus and 105 patients treated with Chhabra shunt after unsuccessful ETV.
|
Class II: prospective, randomized study. This study was downgraded from a Class I to a Class II due to nonblinded outcome assessors
|
No difference between two groups in incidence of shunt malfunction, shunt migration, wound complication, or death. |
|
Gruberet al., 1984
|
Experiences with the anti-siphon device (ASD) in shunt therapy of pediatric hydrocephalus
|
Retrospective review of 41 patients who had primary or secondary placement of an antisiphon device to their shunt system. Comparison of clinical course before and after placement of ASD.
|
Class III: retrospective case series.
|
Fewer shunt malfunctions after ASD placement. Complication rate per patient was four times less frequent and annual ventricular catheter obstruction rate per patient improved 12 times. No statistical analysis to determine significance. |
|
Kan eta l., 2007
|
Predicting slitlike ventricles in children on the basis of baseline characteristics at the time of shunt insertion
|
244 children who underwent shunt placement with either a differential pressure valve, Delta valve or Orbis-Sigma valve and had one year follow-up data were reviewed for development of slit like ventricles.
|
Class III: retrospective review
|
23 patients developed slitlike ventricles: 10.8% of differential pressure valves, 10.5% of Delta valves, and 3.6% of Orbis-Sigma valves p=0.007. Children with DP or Delta valve were 1.66 times more likely to develop slitlike ventricles than with an Orbis-Sigma valve. |
|
Mangano et al., 2005
|
Early programmable valve malfunctions in pediatric hydrocephalus
|
189 children who underwent shunt placement with either a programmable valve (Strata or Codman-Medos with ASD) or a non-programmable valve (PS Medical) were retrospectively analyzed for time to shunt malfunction and CSF protein levels.
|
Class III: retrospective cohort review, short follow-up (mean=9 months)
|
Programmable valves had higher rate of malfunction (11.1% compared to 0%) but did not reach statistical significance. |
|
Miranda et al., 2011
|
Initial Proximal Obstruction of Ventriculoperitoneal Shunt in Patients with Preterm-Related Posthaemorrhagic Hydrocephalus
|
Retrospective review of shunt survival in 103 patients treated for preterm-related post-hemorrhagic hydrocephalus.
|
Class III: retrospective review
|
42 episodes of obstruction. Fixed medium pressure valves were associated with a higher rate of obstruction compared to low pressure valves, only statistically significant in those patients with a weight over 2000 g, p=0.040. |
|
Ramadwar et al., 1997
|
Infantile Hydrocephalus: A Comparison of the Delta Valve and Multipurpose Valve
|
28 patients underwent retrospective comparison of the efficacy of the Delta valve versus the multipurpose (Heyer-Shulte) valve.
|
Class III: retrospective review. Small sample.
|
69% of patients with Delta valve and 53% of patients with Multipurpose valve required revision. Did not reach statistical significance. |
|
Robinson et al., 2002
|
Outcome Analysis of Initial Neonatal Shunts: Does the Valve Make a Difference?
|
158 patients retrospectively analyzed for significant factors associated with shunt malfunction.
|
Class III: retrospective case series
|
Revision rate per year was 4 times higher for patients with no valve or low-pressure valve (72% 5-year failure rate) than for patients with medium or high pressure valve (47% 5-year failure rate). p=0.0005 |
|
Sainte-Rose et al., 1991
|
Mechanical Complications in Shunts
|
Retrospective review of the mechanical complications leading to shunt malfunction in 1,719 patients with shunted hydrocephalus. Patients were treated with distal slit valves or medium pressure proximal valves.
|
Class III: retrospective case series
|
A higher risk of proximal occlusion is associated with flanged ventricular catheters (p<0.04); shunts with proximal medium pressure valves are less likely to malfunction than shunts with distal slit valves (p<0.000002); open-ended distal catheters associated with fewer distal obstructions (p<0.0003). |
|
Smely C, Velthoven V 1997
|
Comparative Study of Two Customary Cerebrospinal Fluid Shunting Systems in Early Childhood Hydrocephalus
|
Retrospective review of 66 infants treated with CordisOrbis-Sigma Valve compared to 53 children treated with Codman Holter Valve ventriculo-atrial system.
|
Class III: retrospective cohort review
|
Codman Holter Valve group demonstrated a greater than double risk of shunt complications in comparison to the ventriculoperitoneal Orbis-Sigma valve system. 48.5% of patients with OSV required one or more revisions, 98.1% of patients with HV required one or more revisions (p<0.001). |
|
Tuli et al., 2000
|
Risk factors for repeated cerebrospinal shunt failures in pediatric patients with hydrocephalus
|
Data prospectively collected on 839 patients undergoing primary shunt insertion. 1183 episodes of shunt failure occurred. Valve types included flow regulated and differential pressure.
|
Class III: prospective cohort study, post-hoc analysis
|
No evidence of an association between shunt malfunction and type of shunt hardware. |
|
Virella et al., 2002
|
Distal slit valve and clinically relevant CSF overdrainage in children with hydrocephlus
|
Retrospective review of 101 patients undergoing shunt placement with a distal slit valve or a Delta level 1 valve with antisiphon component.
|
Class III: retrospective case series
|
No significant differences were found between the DSV and Delta with AS groups in number of revisions, infections, or over drainage.. |
|
Kaiser et al., 1997
|
Conventional Versus Delta Valve in the Treatment of Hydrocephalus in Early Infancy
|
Prospective study comparing conventional medium valve with Delta level 1 valve in 25 infants younger than 6 months.
|
Class III: prospective randomized. Poor description of study design and data.
|
No difference in number of revisions. Fewer proximal revisions in Delta Valve group. No determination of significance from described data. |
|
Serlo et al., 1986
|
Ball and Spring or Slit and Core Valve for Hydrocephalus Shunting?
|
Retrospective review of consecutive series of 148 children treated with shunting procedures with either the Pudenz-Heyer valve or the Hakim-Cordis valve.
|
Class III: retrospective review
|
No significant differences in efficacy. Tendency towards increased rate of catheter rupture in Pudenz-Heyer valve and increased rate of slit ventricles in Hakim-Cordis valve. The higher patency rate of the Pudenz-Heyer valve was statistically significant (p<0.001). |
*ASD = antisiphon device; DPV = differential pressure valve; ETV = endoscopic third ventriculostomy; pt = patient; VP = ventriculoperitoneal.
Table 2. New evidence included in 2020 Update
| Author |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
Riva-Cambrin et al, 2016
|
A prospective observational study to determine risk factors for shunt failure.
|
II
|
This study determined that valve type had no impact on shunt survival. |
|
Beez et al, 2014
|
A retrospective study of patients <16 comparing paediGav and Codman Hakim valves. |
II
|
This study showed that valve type did not influence the risk of shunt failure. |
|
Beuriat et al, 2017
|
Retrospective review of children treated for hydrocephalus.
|
III
|
This study demonstrated the significant impact of valve type on shunt survival. |
|
Hatlen et al,
|
A retrospective study of patients with CSF shunt insertion.
|
III
|
This study demonstrated the significant impact of valve type on shunt survival. |
|
Alavi et al,
|
A retrospective study of patients who received a valve exchange towards an adjustable differential pressure valve with gravitational unit. |
III
|
This study demonstrated the significant impact of valve type on shunt survival. |
|
Kahilogullari et al, 2018
|
A retrospective study of patients who had shunt surgery at the time of myelomeningocele repair. |
III
|
This study demonstrated significance for unique outcome variables. |
|
Oushy et al, 2017
|
A retrospective study of infants and neonates who had multifocal intraparenchymal hemorrhages following shunt placement. Ventricular size ratios, laboratory values, clinical presentation, shunt and valve type, and operative timing and approach were analyzed. |
III
|
This study demonstrated significance for unique outcome variables. |
|
References
- Nulsen FE, Spitz EB. Treatment of hydrocephalus by direct shunt from ventricle to jugular vain. Surgical forum. 1951:399-403.
- Ahn ES, Bookland M, Carson BS, Weingart JD, Jallo GI. The Strata programmable valve for shunt-dependent hydrocephalus: the pediatric experience at a single institution. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2007;23(3):297-303.
- Arnell K, Eriksson E, Olsen L. The programmable adult Codman Hakim valve is useful even in very small children with hydrocephalus. A 7-year retrospective study with special focus on cost/benefit analysis. Eur J Pediatr Surg. 2006;16(1):1-7.
- Breimer GE, Sival DA, Hoving EW. Low-pressure valves in hydrocephalic children: a retrospective analysis. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2012;28(3):469-473.
- Guthkelch AN. The treatment of infantile hydrocephalus by the Holter valve. Br J Surg. 1967;54(8):665-673.
- Haberl EJ, Messing-Juenger M, Schuhmann M, et al. Experiences with a gravity-assisted valve in hydrocephalic children. Clinical article. Journal of neurosurgery Pediatrics. 2009;4(3):289-294.
- Hahn YS. Use of the distal double-slit valve system in children with hydrocephalus. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1994;10(2):99-103.
- Hanlo PW, Cinalli G, Vandertop WP, et al. Treatment of hydrocephalus determined by the European Orbis Sigma Valve II survey: a multicenter prospective 5-year shunt survival study in children and adults in whom a flow-regulating shunt was used. Journal of neurosurgery. 2003;99(1):52-57.
- Hertle DN, Tilgner J, Fruh K, et al. Reversible occlusion (on-off) valves in shunted tumor patients. Neurosurgical review. 2010;34(2):235-242.
- Hoekstra A. Artificial shunting of cerebrospinal fluid. The International journal of artificial organs. 1994;17(2):107-111.
- Kestle JR, Walker ML. A multicenter prospective cohort study of the Strata valve for the management of hydrocephalus in pediatric patients. Journal of neurosurgery. 2005;102(2 Suppl):141-145.
- Martinez-Lage JF, Almagro MJ, Del Rincon IS, et al. Management of neonatal hydrocephalus: feasibility of use and safety of two programmable (Sophy and Polaris) valves. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2008;24(5):549-556.
- Mauer UM, Kunz U. More malfunctioning Medos Hakim programmable valves: cause for concern? Journal of neurosurgery. 2011;115(5):1047-1052.
- Meling TR, Egge A, Due-Tonnessen B. The gravity-assisted Paedi-Gav valve in the treatment of pediatric hydrocephalus. Pediatric neurosurgery. 2005;41(1):8-14.
- Piatt JH, Jr., Carlson CV. A search for determinants of cerebrospinal fluid shunt survival: retrospective analysis of a 14-year institutional experience. Pediatric neurosurgery. 1993;19(5):233-241; discussion 242.
- Jain H, Natarajan K, Sgouros S. Influence of the shunt type in the difference in reduction of volume between the two lateral ventricles in shunted hydrocephalic children. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2005;21(7):552-558.
- Keen J. Casey Holter and the Spitz-Holter valve. Eur J Pediatr Surg. 1992;2 Suppl 1:5-6.
- Kondageski C, Thompson D, Reynolds M, Hayward RD. Experience with the Strata valve in the management of shunt overdrainage. Journal of neurosurgery. 2007;106(2 Suppl):95-102.
- Nomura S, Fujii M, Kajiwara K, et al. Factors influencing spinal canal stenosis in patients with long-term controlled hydrocephalus treated with cerebrospinal fluid shunt. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2010;26(7):931-935.
- Miyake H, Ohta T, Kajimoto Y, Ogawa D. A clinical survey of hydrocephalus and current treatment for hydrocephalus in Japan: analysis by nationwide questionnaire. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1999;15(8):363-368.
- Moritake K, Nagai H, Miyazaki T, et al. Analysis of a nationwide survey on treatment and outcomes of congenital hydrocephalus in Japan. Neurologia medico-chirurgica. 2007;47(10):453-460; discussion 460-451.
- Drake JM, Kestle JR, Milner R, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998;43(2):294-303; discussion 303-295.
- Kestle J, Drake J, Milner R, et al. Long-term follow-up data from the Shunt Design Trial. Pediatric neurosurgery. 2000;33(5):230-236.
- Davis SE, Levy ML, McComb JG, Sposto R. The delta valve: how does its clinical performance compare with two other pressure differential valves without antisiphon control? Pediatric neurosurgery. 2000;33(2):58-63.
- Hatlen TJ, Shurtleff DB, Loeser JD, Ojemann JG, Avellino AM, Ellenbogen RG. Nonprogrammable and programmable cerebrospinal fluid shunt valves: a 5-year study. Journal of neurosurgery Pediatrics. 2012;9(5):462-467.
- Jain H, Sgouros S, Walsh AR, Hockley AD. The treatment of infantile hydrocephalus: "differential-pressure" or "flow-control" valves. A pilot study. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2000;16(4):242-246.
- Khan RA, Narasimhan KL, Tewari MK, Saxena AK. Role of shunts with antisiphon device in treatment of pediatric hydrocephalus. Clinical neurology and neurosurgery. 2010;112(8):687-690.
- Liniger P, Marchand S, Kaiser GL. Flow control versus antisiphon valves: late results concerning slit ventricles and slit-ventricle syndrome. Eur J Pediatr Surg. 2003;13 Suppl 1:S3-6.
- Mangano FT, Menendez JA, Habrock T, et al. Early programmable valve malfunctions in pediatric hydrocephalus. Journal of neurosurgery. 2005;103(6 Suppl):501-507.
- McGirt MJ, Buck DW, 2nd, Sciubba D, et al. Adjustable vs set-pressure valves decrease the risk of proximal shunt obstruction in the treatment of pediatric hydrocephalus. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2007;23(3):289-295.
- Notarianni C, Vannemreddy P, Caldito G, et al. Congenital hydrocephalus and ventriculoperitoneal shunts: influence of etiology and programmable shunts on revisions. Journal of neurosurgery Pediatrics. 2009;4(6):547-552.
- Pollack IF, Albright AL, Adelson PD. A randomized, controlled study of a programmable shunt valve versus a conventional valve for patients with hydrocephalus. Hakim-Medos Investigator Group. Neurosurgery. 1999;45(6):1399-1408; discussion 1408-1311.
- Smely C, Van Velthoven V. Comparative study of two customary cerebrospinal fluid shunting systems in early childhood hydrocephalus. Acta neurochirurgica. 1997;139(9):875-881; discussion 882.
- Warf BC. Comparison of 1-year outcomes for the Chhabra and Codman-Hakim Micro Precision shunt systems in Uganda: a prospective study in 195 children. Journal of neurosurgery. 2005;102(4 Suppl):358-362.
- Gruber R, Jenny P, Herzog B. Experiences with the anti-siphon device (ASD) in shunt therapy of pediatric hydrocephalus. Journal of neurosurgery. 1984;61(1):156-162.
- Virella AA, Galarza M, Masterman-Smith M, Lemus R, Lazareff JA. Distal slit valve and clinically relevant CSF overdrainage in children with hydrocephalus. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2002;18(1-2):15-18.
- Kaiser GL, Horner E, Marchand S, Jost A. Conventional versus Delta valve in the treatment of hydrocephalus in early infancy. Eur J Pediatr Surg. 1997;7 Suppl 1:45-46.
- Kan P, Walker ML, Drake JM, Kestle JR. Predicting slitlike ventricles in children on the basis of baseline characteristics at the time of shunt insertion. Journal of neurosurgery. 2007;106(5 Suppl):347-349.
- Miranda P, Simal JA, Menor F, Plaza E, Conde R, Botella C. Initial proximal obstruction of ventriculoperitoneal shunt in patients with preterm-related posthaemorrhagic hydrocephalus. Pediatric neurosurgery. 2011;47(2):88-92.
- Robinson S, Kaufman BA, Park TS. Outcome analysis of initial neonatal shunts: does the valve make a difference? Pediatric neurosurgery. 2002;37(6):287-294.
- Sainte-Rose C, Piatt JH, Renier D, et al. Mechanical complications in shunts. Pediatric neurosurgery. 1991;17(1):2-9.
- Tuli S, Drake J, Lawless J, Wigg M, Lamberti-Pasculli M. Risk factors for repeated cerebrospinal shunt failures in pediatric patients with hydrocephalus. Journal of neurosurgery. 2000;92(1):31-38.
- Ramadwar RH, Carachi R, Young DG. Infantile hydrocephalus: a comparison of the Delta valve and multipurpose valve. Eur J Pediatr Surg. 1997;7 Suppl 1:44-45.
- Serlo W, von Wendt L, Heikkinen ES, Heikkinen ER. Ball and spring or slit and core valve for hydrocephalus shunting? Annals of clinical research. 1986;18 Suppl 47:103-106.
- Riva-Cambrin J, Kestle JR, Holubkov R, et al. Risk factors for shunt malfunction in pediatric hydrocephalus: a multicenter prospective cohort study. Journal of neurosurgery Pediatrics. 2016;17(4):382-390.
- Beez T, Sarikaya-Seiwert S, Bellstadt L, Muhmer M, Steiger HJ. Role of ventriculoperitoneal shunt valve design in the treatment of pediatric hydrocephalus--a single center study of valve performance in the clinical setting. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2014;30(2):293-297.
- Beuriat PA, Puget S, Cinalli G, et al. Hydrocephalus treatment in children: long-term outcome in 975 consecutive patients. Journal of neurosurgery Pediatrics. 2017;20(1):10-18.
- Alavi S, Schulz M, Schaumann A, Schwarz K, Thomale UW. Valve exchange towards an adjustable differential pressure valve with gravitational unit, clinical outcome of a single-center study. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2017;33(5):759-765.
- Kahilogullari G, Etus V, Guler TM, Karabagli H, Unlu A. Does Shunt Selection Affect the Rate of Early Shunt Complications in Neonatal Myelomeningocele-Associated Hydrocephalus? A Multi-Center Study. Turkish neurosurgery. 2018;28(2):303-306.
- Oushy S, Parker JJ, Campbell K, et al. Frontal and occipital horn ratio is associated with multifocal intraparenchymal hemorrhages in neonatal shunted hydrocephalus. Journal of neurosurgery Pediatrics. 2017;20(5):432-438.