J Neurosurg Pediatrics (Suppl) 14:77–81, 2014
AANS, 2014
(Original text of the guideline was edited to reflect the update. Please click here for the original publication.)
Pediatric hydrocephalus: systematic literature review and evidence-based guidelines
Part 10: Change in ventricle size as a measurement of effective treatment of hydrocephalus
UPDATE
Dimitrios C. Nikas, MD1,2, Alexander F. Post, MD3, Asim F. Choudhri, MD4,5, Catherine A. Mazzola, MD6, Laura Mitchell, MA7 and Ann Marie Flannery8
1Department of Neurosurgery, University of Illinois at Chicago, Chicago, Illinois; 2Advocate Children’s Hospital, Oak Lawn, Illinois; 3Division of Pediatric Neurological Surgery, Department of Neurosciences and Pediatrics, Goryeb Children’s Hospital–Morristown Medical Center, Morristown, New Jersey; 4Departments of Radiology, Ophthalmology, and Neurosurgery, University of Tennessee Health Science Center, and 5Le Bonheur Neuroscience Institute, Le Bonheur Children’s Hospital, Memphis, Tennessee; 6Division of Pediatric Neurological Surgery, Goryeb Children’s Hospital, Morristown, New Jersey; 7Congress of Neurological Surgeons, Schaumburg, Illinois; 8Department of Neurological Surgery, Saint Louis University, St. Louis, Missouri
Object. The objective of this systematic review is to answer the following question: Does ventricle size after treatment have a predictive value in determining the effectiveness of surgical intervention in pediatric hydrocephalus? Methods. The US National Library of Medicine PubMed/MEDLINE database and the Cochrane Database of Systematic Reviews were searched using MeSH headings and key words relevant to change in ventricle size after surgical intervention for hydrocephalus in children. An evidentiary table was assembled summarizing the studies and the quality of evidence (Classes I–III).
Results. Six articles satisfied inclusion criteria for the evidentiary tables for this part of the guidelines. All were Class III retrospective studies.
Conclusions. Recommendation: There is insufficient evidence to recommend a specific change in ventricle size as a measurement of the effective treatment of hydrocephalus and as a measurement of the timing and effectiveness of treatments including ventriculoperitoneal shunts and third ventriculostomies. Strength of Recommendation: Level III, unclear clinical certainty.
(http://thejns.org/doi/abs/10.3171/2014.7.PEDS14330)
Keywords: hydrocephalus, ventricle size, evidence-based guidelines, practice guidelines
Abbreviations used in this paper: AANS = American Association of Neurological Surgeons; CNS = Congress of Neurological Sur- geons; CPC = choroid plexus cauterization; ETV = endoscopic third ventriculostomy; FOR = frontal and occipital horn ratio; VP = ventriculoperitoneal.
The decision to treat hydrocephalus with an external shunt or endoscopic third ventriculostomy (ETV) is based on a variety of factors. The determination of successful outcome rests on a multitude of clinical and imaging correlates. Imaging indicators that have been examined include ventricle size, presence of a flow void in the ETV site, amount of CSF over the cerebral hemispheres, and the degree of periventricular edema. The objective of this particular systematic review is to answer the question: Does ventricle size after treatment have a predictive value for effectiveness of surgical intervention in pediatric hydrocephalus?
Ventricle size before and after intervention is a readily available measurement that has been used to assess success or failure of treatment. Particular attention has been given to changes in ventricle size after ETV.1-3
Correlation with neurodevelopmental sequelae as well as correlation with other imaging parameters, such as presence of flow voids after ETV, have been suggested as indications of successful interventions.4-7 The evaluation of the effectiveness of treatment has therefore been limited because developmental outcomes are most applicable in infants and younger children and flow voids observed on MR images are applicable only to ETV treatment. This review, therefore, focuses on ventricle size as a tool, albeit a limited one, in the evaluation of the effectiveness of treatment. Ventricle size is an outcome that could be used to assess patients of all ages as well as both forms of intervention—the ventriculoperitoneal (VP) shunt and the ETV. The purpose of this evidence-based review is to critically examine data from the literature pertaining to change of ventricle size as a predictor of the success of surgical intervention.
Methods
We searched the US National Library of Medicine (PubMed/MEDLINE) and the Cochrane Database of Systematic Reviews for the period January 1966 through March 2012 using strategies listed below. The inclusion and exclusion criteria adhered to the protocol outlined in the methods section, Part 1, of these evidence-based guidelines.8
Search Terms
PubMed/MEDLINE
- “Cerebrospinal Fluid Shunts”[MeSH] AND “Hy- drocephalus”[Majr:noexp]
- 1 AND (“Magnetic Resonance Imaging”[MeSH] OR “Tomography, X-Ray Computed”[MeSH] OR Ultra- sonography[MeSH] OR imaging[tiab])
- 2 AND (“ventricular size”[TIAB] OR “ventricular dilation”[tiab] OR ventricle[tiab] OR ventricles[tiab])
- Limit to Child (0–18 years)
- Limit to English and Humans
- Limit 3 to Child (0–18 years)
- Limit to English and Humans Number = 81
Cochrane Database
- MeSH descriptor Child
- MeSH descriptor Infant
- MeSH descriptor Hydrocephalus
- MeSH descriptor Cerebrospinal Fluid Shunts
- (MeSH descriptor Magnetic Resonance Imaging) or (MeSH descriptor Ultrasonography) or (MeSH de- scriptor Tomography, X-Ray Computed) or imaging
- (1 or 2) and 3 and 4 and 5
- 3 and 4 and 5
Search Strategy
An evidentiary table was constructed to facilitate data review and analysis by the Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Task Force.
For each article included in the evidentiary table, the study type, summary findings, and major conclusions were recorded, and a preliminary data class was assigned. The Task Force met to discuss the ranking of the evidence and the classification of data. Recommendations then were made based on the strength of the data in the evidentiary table. In these discussions, if a disagreement was encountered among members, a blinded vote was held and a consensus or majority opinion was reached.
Authors performed an updated literature search (in PubMed and Cochrane Central) for this guideline chapter through a medical librarian at the Congress of Neurological Surgeons Guidelines office using the below-mentioned existing search terms to update the original search through November 30, 2019.
Search Results
A total of 81 abstracts were screened and 18 full-text articles listed in the databases were retrieved for review (Fig. 1). The selection for review was based on the determination of evidence data relevant to the question of the effect of treatment on ventricle size. An examination of the reference lists of these 18 full-text articles yielded 4 additional articles that warranted full-text review. All 22 articles were read and reviewed in detail by the full Task Force. Sixteen articles were excluded based on predefined criteria, which are described in Part 18 of the Guidelines. Six articles satisfied the inclusion criteria and form the basis for the evidentiary tables in this recommendation.
An additional 3 studies out of the 70 yielded by the 2020 update met inclusion criteria from the original guideline and were included (Figure 2).
Results
The 6 articles that met the inclusion criteria and were selected for final review were all Class III retrospective studies (Table 1).
In 2000, Kulkarni et al2 published the results of a retrospective, blinded observational study of a group of 29 children who had undergone ETV and whose ventricle size was assessed by 4 independent observers using the FOR (frontal and occipital horn ratio) both preoperatively and postoperatively (Table 1). Postoperatively, the mean reduction in ventricle size was 7% in cases that were deemed treatment failures (8 patients in whom symptoms either recurred or never resolved) and 16% in cases that were clinically successful (21 patients)—a result that was statistically significant (p = 0.03, t-test). The authors concluded that ventricle size appeared to be somewhat reduced in both groups of patients; however, the reduction was significantly greater among the clinically successful cases. The authors also assessed imaging correlates; they found that the presence of a flow void seemed to correlate with clinical success and its absence with clinical failure. The most significant limitation in this study is its retrospective nature. Surgeon bias, the linear measurement of ventricle size, and the variation in time when postoperative imaging was conducted were confounding factors.
In another retrospective study, published in 2009, Warf et al8 described neurocognitive outcomes and ventricle volumes in infants with myelomeningoceles and hydrocephalus (Table 1). The same method was used to compare ventricle size in 55 children treated by ETV with choroid plexus cauterization (CPC), 19 children who received a VP shunt, and another 19 children who required no intervention. The mean FOR was similar among groups, with no significant difference between the untreated group and either the VP shunt or ETV with CPC group before treatment. The groups also were compared for neurocognitive outcomes, and no significant difference was identified. The FOR did not correlate with neurocognitive performance. Bias existed with respect to the VP shunt group because most patients had already experienced ETV with CPC failure. This is another non- randomized study with the potential for strong confounding factors within treatment groups. A valid conclusion was that stable mild-to-moderate ventriculomegaly alone should not trigger intervention in an asymptomatic infant with a myelomeningocele.
Another Class III single-center retrospective study in the myelomeningocele population was published by Chakraborty et al9 in 2008 (Table 1). These authors studied 28 patients who were selected from a group of 54 who satisfied the determined inclusion criteria. Overall, using a stringent shunt placement policy, about half (51.9%) of their patients required a VP shunt. The authors suggest that a more critical evaluation and tolerance of ventriculomegaly may decrease the need for shunt placement and will reduce shunt dependency in children with myelomeningocele, without worsening outcome.
In a study by St. George et al., limited by its small number of subjects, the authors examined 13 patients with multiple diagnoses who had undergone ETV (Table 1).3
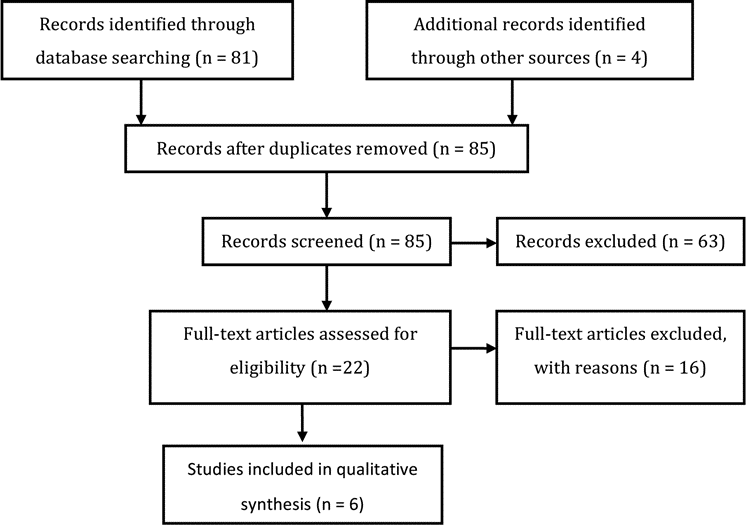
Fig. 1. Flowchart showing the process involved in identifying relevant literature.
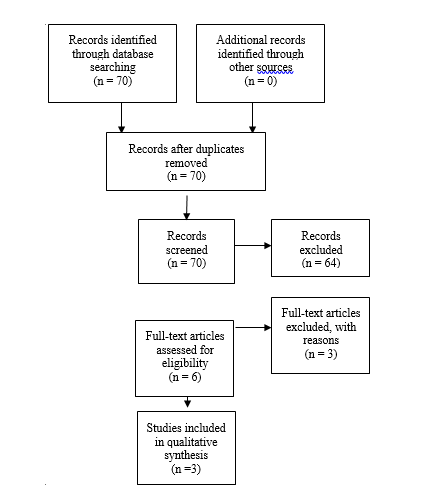
Fig. 2. Flowchart showing the process involved in identifying relevant literature for the 2020 Update The criteria for “records excluded” and “full text articles excluded with reasons” are detailed in Part 1 of the Guidelines.
The comparison of preoperative and postoperative ventricular volume was confined to patients in this treatment group. The authors found postoperative measurements of ventricular volume to be lower than measurements obtained preoperatively but higher than normalized values for patient ages and sexes. The pattern of change in ventricle size varied between a large ventricular volume group, which demonstrated a significant decrease at the 3- to 6-month postoperative time point, and a small volume group, in which there was a much less steep reduction in ventricle size in the first 3–6 months. After that time period, the volume appeared to stabilize or fall slightly.
Two additional papers reported that ventricle size was not necessarily a predictor of outcome (Table 1). These 2 studies looked at ETV alone and found that in both infants and older children, reduction in ventricle size was not necessary to have a clinically effective treatment of hydrocephalus. Like the previously cited studies, these studies were retrospective, and both enrolled a small number of patients (Buxton et al.: n = 27; Kim et al.: n = 29 in whom neuroimaging studies were available).4,10
2020 Update
Di Rocco et al11 presented Class III data indicating that all successful ETV cases had a progressive reduction in ventricular volume (as well as increase in volume of subarachnoid spaces). Pindrik et al12, in a class III study, applied measurements of 3rd ventricle (width and mid-sagittal cross-sectional area) and report that these respond more to a successful ETV than lateral ventricular measurements. Romeo et al13 report reduction in ventricular size in patients with tectal plate gliomas whose hydrocephalus was treated with ETV – with the most significant ventricular size reduction observed in the 1-year follow-up (class III). Because of the small cohort of patients (22) in combination with the retrospective design and the specific etiology of hydrocephalus, these studies cannot substantially change the original recommendations.
Excluded Evidence
The evidence reviewed was predominantly Class III. Those papers not used in the analysis were excluded for multiple reasons including the following: reports combining adult and pediatric patient populations without reporting pediatric patients separately;4,5,14-17 a review of other studies;18 and a technical report.19 Jain et al20 reported the effect of valve design on repeated operation, not on effectiveness of treatment. Another paper described how to measure ventricles.21 Kombogiorgas et al22 confined their evaluation to the question of ventricle size predicting the need for shunting after tumor resection in patients with posterior fossa tumors. The large study by Shankaran and colleagues23 looked at the impact of vetriculomegaly on outcome, without expressly addressing the shunt status of the patients and was thus excluded. One article could not be retrieved for full-text review and was therefore excluded.24 The paper by Horbar et al25 did not meet inclusion criteria due to enrollment of fewer than 10 patients. One by Choudhury26 was excluded because it did not report ventricle size as an outcome of interest and therefore did not report data to answer the clinical questions addressed in this section. Goumnerova and Frim5 reported a study including adults and children, but did not analyze the pediatric patients separately.
Conclusions
Recommendation: There is insufficient evidence to recommend a specific change in ventricle size as a measurement of effective treatment of hydrocephalus and as a measurement of the timing and effectiveness of treatments including ventriculoperitoneal shunts and third ventriculostomies. Strength of Recommendation: Level III, unclear clinical certainty.
The purpose of hydrocephalus treatment is to return the CSF and/or pressure within the brain of an affected infant or child to as normal a condition as possible. Resources to effect these changes are currently limited primarily to the use of a ventriculoperitoneal (VP) shunt or endoscopic third ventriculostomy (ETV). Intracranial pressure and its effect on brain function cannot easily be measured, and thus an alternate way of judging treatment effectiveness is necessary. The size of ventricles revealed by a number of imaging modalities, including ultrasonography, CT, and MR imaging, is frequently used as a measure of the effectiveness of intervention. Our evaluation of the evidence reveals that reliance on ventricle size alone, as a demonstration of treatment effectiveness, is not supported by the available evidence. Unfortunately, there are no other direct methods currently in general use. Certainly, developmental progress is worth monitoring, but this is more difficult to accomplish for routine post- surgical evaluations.
This systematic review and evidence-based guideline demonstrates that the objective measurement of ventricle size by simple methods such as the frontal and occipital horn ratio (FOR) has not proven to be a reliable bench- mark of effectiveness. Clinical outcome ascertained by the neurosurgeon and team is still the most accepted and useful evaluation, surpassing other more “objective” standards.
Acknowledgments
We acknowledge the American Association of Neurological Surgeons (AANS)/Congress of Neurological Surgeons (CNS) Joint Guidelines Committee for the members’ reviews, comments, and suggestions; Laura Mitchell, Guidelines Project Manager for the CNS, for her contributions; Pamela Shaw, research librarian, for her assistance with the literature searches; Kevin Boyer for his assistance with data analysis; and Sue Ann Kawecki for her assistance with editing. We also acknowledge the following peer reviewers for their contributions to review the update to the guidelines: Jennifer Sweet, MD, Brandon Rocque, MD, Christoph Greissenauer, MD, Jeffrey Olson, MD.
Disclosure
The systematic review and evidence-based guidelines were funded exclusively by the CNS and AANS Pediatric Section, which received no funding from outside commercial sources to support the development of this document.
Conflict(s) of Interest: None. All Task Force members declared any potential conflicts of interest prior to beginning work on this evidence review.
Conflict(s) of Interest: None. All Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Update Task Force members declared any potential conflicts of interest prior to beginning work on this systematic review and evidence-based guidelines.
Author contributions to the study and manuscript preparation include the following. Conception and design: AANS/CNS Joint Section on Pediatrics. Acquisition of data: all authors. Analysis and interpretation of data: all authors. Drafting the article: all authors. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manu- script on behalf of all authors: Flannery. Administrative/technical/ material support: all authors. Study supervision: Flannery.
Table 1. Ventricular Size Evidence Table
| Author |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
Chakraborty et al., 2008
|
Single center, 10 year retrospective review of MMC pts, excluded patients closed at OSH. Evaluated shunt insertion, complications, and clinical outcomes. Shunt placed for symptomatic hydrocephalus or severe hydrocephalus, or progression of ventriculomegaly after closure. 54 cases included, 28 (52%) required a shunt
|
Class III, single center, retrospective, single cohort, “Increasing ventriculomegaly assessed by a neuroradiologist with the neurosurgeron. No specific imaging measurement was used to define hydrocephalus”
|
Shunt rates in MMC lower than previously published results (and similar to that of in-utero closure) when allowing mild ventriculomegaly |
|
Kim et al., 2000
|
32 children with ETV. Mean follow-up: 19 months.
28 had neuroimaging
Two groups: Good (21) and poor (8) outcome. Ventricular size, edema, widening of SA space, surgical changes in the IIIv. Floor, cine-MR findings studied between the 2 groups. |
Class III: Retrospective
|
Good outcomes group:
- 11/16 patients had decrease in ventricular size one month postop.
- 5/16 patients minimal changes only
- Ventricular size tended to decrease with time
Changes in ventricular size could not predict surgical outcome completely in themselves. No correlation |
|
|
St George et al., 2004
|
Single center, 13 consecutive hydrocephalic patients undergoing ETV studied, MRI scans reviewed and ventricular volume calculated. Preop volume 207cc, one week 120 cc, three months 104cc, six months 119 cc, 12 months 146 cc, 24 months 185 cc (skewed because they didn’t have as many 24 month follow-ups so it was overly influenced by increase in patients 13 who had big vents to start with). Decrease in size was more rapid in those with larger vents
|
Class III, single center, no comparison group (didn't compare using volumetric evaluation of shunt patients)
|
Patients with moderate ventriculomegaly had a less steep decrease in vent size in first 6 months after ETV than those with large preop vents. Steady-state volumes were larger than normal |
|
|
Warf et al., 2009
|
93 patients, spina bifida
Developmental assessment, and vent size non-random, non-controlled
55 ETV CPC19 VPS, 19 no Tx
|
Class III: retrospective
|
No Tx better development than treated, better receptive communication in ETV c/w VPS(p=0.02)
No difference in vent volume between ETV and VPS |
|
Buxton et al., 1998
|
Outcome and reasons for failure in ETVs performed in children <1year old: 27 total patients.
Postop ventricular size and flow through stoma documented. Comparison was performed between successes and failures (21 patients -77%).
|
Class III: Retrospective review of Prospectively acquired data
|
Postop size (table 2) was not an indicator of success or failure. Size does not matter
|
|
Kulkarni et al., 2000
|
Retrospective FOR (measure of ventricular size) change post ETV comparing successes and failures
|
Class III: Retrospective case series
Images evaluated by two observers in terms of ventricular size, flow void, periventricular edema, and CSF in SA space.
Comparison with two-tailed t-test
|
ETV failures; mean postop reduction in ventricular size: 7%
ETV clinically successful: 16%
(reduction significantly greater)
All had decreased FOR, successes slightly more but not to normal (vents do not return to normal even after successful ETV)
|
|
Table 2. New evidence included in 2020 Update
| Author |
Study Description |
Data Class, Quality and Reasons |
Results and Conclusions |
|
Di Rocco et al, 2012
|
Prospective study that analyzed the changes in cerebrospinal fluid (CSF) distribution after endoscopic third ventriculocisternostomy (ETV).
|
III
|
Results indicate that all successful ETV cases had a progressive reduction in ventricular volume (as well as increase in volume of subarachnoid spaces). |
|
Pindrik et al, 2013
|
Retrospectively reviewed applied measurements of 3rd ventricle (width and mid-sagittal cross-sectional area). |
III
|
This study showed that these respond more to a successful ETV than lateral ventricular measurements. |
|
|
Romeo et al, 2013
|
Retrospective study of change in ventricular size after ETV for TPGs.
|
III
|
This study reported reduction in ventricular size in patients with tectal plate gliomas whose hydrocephalus was treated with ETV – with the most significant ventricular size reduction observed in the 1-year follow-up. |
|
|
References
- Cinalli G, Spennato P, Ruggiero C, et al. Intracranial pressure monitoring and lumbar puncture after endoscopic third ventriculostomy in children. Neurosurgery. 2006;58(1):126-136; discussion 126-136.
- Kulkarni AV, Drake JM, Armstrong DC, Dirks PB. Imaging correlates of successful endoscopic third ventriculostomy. Journal of neurosurgery. 2000;92(6):915-919.
- St George E, Natarajan K, Sgouros S. Changes in ventricular volume in hydrocephalic children following successful endoscopic third ventriculostomy. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2004;20(11-12):834-838.
- Buxton N, Turner B, Ramli N, Vloeberghs M. Changes in third ventricular size with neuroendoscopic third ventriculostomy: a blinded study. Journal of Neurology, Neurosurgery and Psychiatry. 2002;72(3):385-387.
- Goumnerova LC, Frim DM. Treatment of hydrocephalus with third ventriculocisternostomy: outcome and CSF flow patterns. Pediatric neurosurgery. 1997;27(3):149-152.
- Jack CR, Jr., Kelly PJ. Stereotactic third ventriculostomy: assessment of patency with MR imaging. AJNR American journal of neuroradiology. 1989;10(3):515-522.
- Wilcock DJ, Jaspan T, Worthington BS, Punt J. Neuro-endoscopic third ventriculostomy: evaluation with magnetic resonance imaging. Clinical radiology. 1997;52(1):50-54.
- Warf B, Ondoma S, Kulkarni A, et al. Neurocognitive outcome and ventricular volume in children with myelomeningocele treated for hydrocephalus in Uganda. Journal of neurosurgery Pediatrics. 2009;4(6):564-570.
- Chakraborty A, Crimmins D, Hayward R, Thompson D. Toward reducing shunt placement rates in patients with myelomeningocele. Journal of neurosurgery Pediatrics. 2008;1(5):361-365.
- Kim SK, Wang KC, Cho BK. Surgical outcome of pediatric hydrocephalus treated by endoscopic III ventriculostomy: prognostic factors and interpretation of postoperative neuroimaging. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2000;16(3):161-168; discussion 169.
- Di Rocco F, Grevent D, Drake JM, et al. Changes in intracranial CSF distribution after ETV. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2012;28(7):997-1002.
- Pindrik J, Jallo GI, Ahn ES. Changes in third ventricular size in pediatric patients undergoing endoscopic third ventriculostomy. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2013;29(11):2027-2034.
- Romeo A, Naftel RP, Griessenauer CJ, et al. Long-term change in ventricular size following endoscopic third ventriculostomy for hydrocephalus due to tectal plate gliomas. Journal of neurosurgery Pediatrics. 2013;11(1):20-25.
- Feng H, Huang G, Liao X, et al. Endoscopic third ventriculostomy in the management of obstructive hydrocephalus: an outcome analysis. Journal of neurosurgery. 2004;100(4):626-633.
- Fukuhara T, Vorster SJ, Luciano MG. Risk factors for failure of endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurgery. 2000;46(5):1100-1109; discussion 1109-1111.
- Hommet C, Cottier JP, Billard C, et al. MRI morphometric study and correlation with cognitive functions in young adults shunted for congenital hydrocephalus related to spina bifida. European neurology. 2002;47(3):169-174.
- Jindal A, Mahapatra AK. Correlation of ventricular size and transcranial Doppler findings before and after ventricular peritoneal shunt in patients with hydrocephalus: prospective study of 35 patients. Journal of Neurology, Neurosurgery and Psychiatry. 1998;65(2):269-271.
- Rekate HL. To shunt or not to shunt: hydrocephalus and dysraphism. Clinical Neurosurgery. 1985;32:593-607.
- Schroeder HW, Oertel J, Gaab MR. Endoscopic aqueductoplasty in the treatment of aqueductal stenosis. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2004;20(11-12):821-827.
- Jain H, Natarajan K, Sgouros S. Influence of the shunt type in the difference in reduction of volume between the two lateral ventricles in shunted hydrocephalic children. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2005;21(7):552-558.
- O'Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: A linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatric neurosurgery. 1998;29(5):245-249.
- Kombogiorgas D, Natarajan K, Sgouros S. Predictive value of preoperative ventricular volume on the need for permanent hydrocephalus treatment immediately after resection of posterior fossa medulloblastomas in children. Journal of neurosurgery Pediatrics. 2008;1(6):451-455.
- Shankaran S, Koepke T, Woldt E, et al. Outcome after posthemorrhagic ventriculomegaly in comparison with mild hemorrhage without ventriculomegaly. The Journal of pediatrics. 1989;114(1):109-114.
- Bajpai M, Kataria R, Bhatnagar V, et al. Management of hydrocephalus. Indian journal of pediatrics. 1997;64(6 Suppl):48-56.
- Horbar JD, Walters CL, Philip AG, Lucey JF. Ultrasound detection of changing ventricular size in posthemorrhagic hydrocephalus. Pediatrics. 1980;66(5):674-678.
- Choudhury AR. Avoidable factors that contribute to the complications of ventriculoperitoneal shunt in childhood hydrocephalus. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1990;6(6):346-349.