Guidelines For Pediatric Myelomeningocele
6. The Incidence of Tethered Cord Syndrome in Infants With Myelomeningocele with Prenatal versus Postnatal Repair
Download PDF Neurosurgery, 2019
Sponsored by: Congress of Neurological Surgeons (CNS) and the Section on Pediatric Neurosurgery
Endorsed by: The Congress of Neurological Surgeons (CNS), American Association of Neurological Surgeons (AANS), and Spina Bifida Association (SBA)
Catherine A. Mazzola, MD1, Rachana Tyagi, MD2, Nadege Assassi3, David F. Bauer, MD4, Alexandra D. Beier, DO5, Jeffrey P. Blount, MD6, Susan R. Durham, MD, MS7, Ann Marie Flannery, MD8, Paul Klimo Jr., MD9, Catherine McClung-Smith, MD10, Dimitrios C. Nikas, MD11, Patricia Rehring, MPH12, Mandeep S. Tamber, MD, PhD13
- Goryeb Children’s Hospital, Morristown, New Jersey; Rutgers Department of Neurological Surgery, Newark, New Jersey
- Department of Neurosurgery, Mercer University Medical School, Macon, Georgia
- Department of Surgery, Division of Neurosurgery, Robert Wood Johnson Medical School, New Brunswick, New Jersey
- Department of Surgery, Division of Neurosurgery, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire
- Division of Pediatric Neurosurgery, University of Florida Health Jacksonville, Jacksonville, Florida
- Division of Pediatric Neurosurgery, Department of Neurosurgery, University of Alabama at Birmingham; Children’s of Alabama, Birmingham, Alabama
- The University of Vermont Medical Center, Burlington, Vermont
- Kids Specialty Center, Women’s & Children’s Hospital, Lafayette, Louisiana
- Semmes-Murphey; Department of Neurosurgery, University of Tennessee Health Science Center; Le Bonheur Children’s Hospital, Memphis, Tennessee
- Department of Neurological Surgery, Palmetto Health USC Medical Group, Columbia, South Carolina
- Division of Pediatric Neurosurgery, Advocate Children's Hospital, Oak Lawn, Illinois
- Congress of Neurological Surgeons, Schaumburg, Illinois
- Division of Pediatric Neurosurgery, British Columbia Children’s Hospital, University of British Columbia, Vancouver, British Columbia, Canada
Correspondence:
Catherine A. Mazzola, MD
Goryeb Children’s Hospital and Rutgers New Jersey Medical School
Newark, New Jersey
E-mail: catherine.mazzola@rutgers.edu
Abbreviations:
COI- conflict of interest
HC- hydrocephalus
JAMA- Journal of the American Medical Association
MOMS- Management of Myelomeningocele Study
MM- myelomeningocele
NEJM- New England Journal of Medicine
SB- spina bifida
TCS- tethered cord syndrome
ABSTRACT
Background: There are 1,500 infants born annually in the United States with spina bifida (SB) and myelomeningocele (MM). The incidence of SB in the developing world is much higher because of folic acid deficiency during pregnancy. Recent advances in medicine and technology have made prenatal repair of MM possible.
Objective: The objective of this guideline was to determine if there is a difference in the rate of development of tethered cord syndrome (TCS) in infants who had prenatal closure compared to infants who had MM repair after birth.
Methods: The Guidelines Task Force developed search terms and strategies to search PubMed and Embase for relevant literature published between 1966 and September 2016. Strict inclusion/exclusion criteria were used to screen abstracts and to develop a list of relevant articles for full-text review. Full text articles were then reviewed and, when appropriate, included as evidence.
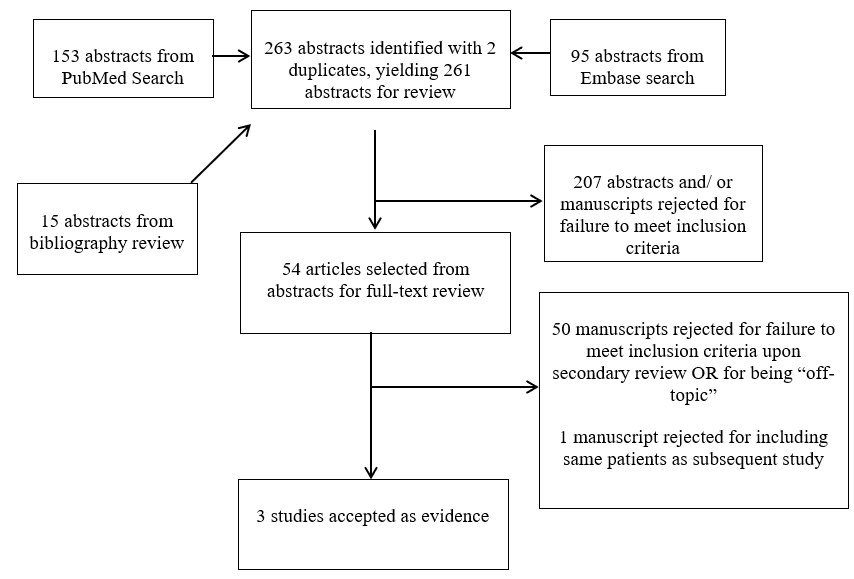
Results: A total of 261 abstracts were identified and reviewed. Fifty-four full text articles were selected for further analysis. Three studies met inclusion criteria.
Conclusions: There was Class II evidence from 1 study and Class III evidence from another 2 studies demonstrating that TCS develops in infants with prenatal MM closure at an equal or higher rate than with postnatal closure. There was an increased risk of development of inclusion cysts in infants who underwent in utero closure. Continued surveillance for TCS and/or the development of inclusion cysts in children with prenatal and postnatal closure of MM is indicated (Level II). Differences between prenatal and postnatal repair with respect to the development of TCS and/or inclusion cysts should be considered alongside other relevant maternal and fetal outcomes when deciding upon a preferred method for MM closure.
Keywords: Fetal, in utero closure, myelomeningocele, postnatal, spina bifida, tethered cord syndrome
RECOMMENDATIONS
PICO Question: Is there a difference in the rate of development of tethered cord syndrome in infants who had prenatal myelomeningocele closure compared to infants who had myelomeningocele closure after birth?
Target Population: Infants and children with myelomeningocele.
Recommendation(s): Continued surveillance for tethered cord syndrome and/or the development of inclusion cysts in children with prenatal and postnatal closure of myelomeningocele is indicated (Level II) as there is evidence that prenatal closure increases the risk of recurrent tethered cord over the baseline rate seen with postnatal closure.
INTRODUCTION
Rationale
Each year, approximately 1,500 infants in the United States are born with spina bifida (SB).1 Data from the National Birth Defects Prevention Network also shows a higher reported incidence in Hispanic women (3.80 per 10,000 live births) than in non-Hispanic black or African-American women (2.73 per 10,000 live births) or women identified as non-Hispanic white (3.09 per 10,000 live births).1 The incidence of SB in developing countries is under-reported, due to the limitations of surveillance data collection by the International Clearinghouse for Birth Defects Surveillance and Research, a voluntary non-profit organization affiliated with the World Health Organization.2 Lower-income countries have a higher prevalence of infants and children with myelomeningocele (MM) and other disabilities than higher-income countries.2 In 2016, Atta et al published an article clearly showing that mandatory legislation enforcing folic acid fortification reduces the incidence of SB, demonstrating that SB is significantly more common in regions without government-mandated folic acid fortification of the food supply.2, 3
Before this guideline, there were no evidence-based guidelines addressing the timing and other variables of MM closure in patients with SB. There are differences in practice regarding the team approach to closure, timing and type of closure techniques, administration of antibiotics, and benefits of amputation vs preservation of the neural placode. Additionally, as technology has advanced, many experts now advocate for in utero (or prenatal) closure of the MM defect. However, the long-term effects of in utero closure have not been well studied. This systematic review was conducted to evaluate all available evidence to aid clinicians and guide clinical practice by determining the best options for management of pediatric MM.
In 1999, in a landmark study published in the Journal of the American Medical Association, Bruner et al reported their success with in utero MM closure in 29 infants.4 They found that there was a decreased need for ventriculoperitoneal shunt placement for hydrocephalus (HC) among infants who underwent prenatal closure of their MM as compared to a historical control (59% vs 91%; p = 0.01). The median age at shunt placement was also older among infants who had prenatal repair (50 vs 5 days; p = 0.006). The authors theorized that the decreased rate of HC may be related to the reduced incidence of hindbrain herniation among study infants (38% vs 95%; p<.001). The National Institutes of Health-sponsored Management of Myelomeningocele Study (MOMS) trial results published in 2011 have shown many benefits of prenatal closure.5 An increased rate of tethered cord syndrome (TCS) was observed in infants who had their MM closed prenatally; however, this increase was not statistically significant.
Many clinicians and researchers advocate for the possibility and advantages of in utero or prenatal repair. It was the intention of the Task Force to investigate and evaluate the literature regarding prenatal repair, specifically in reference to the rate of shunt placement, ambulatory status or motor function and rate of TCS development. The Task Force also aimed to systematically review the literature and make evidence based recommendations about the timing of postnatal closure, as well as to investigate the evidence concerning persistent ventriculomegaly and cognitive impairment. These 5 topics were chosen by consensus to be addressed, because of their importance and relevance.
In this guideline, authors address the incidence of TCS in patients with MM and prenatal vs postnatal repair. For purposes of the literature search, the task force defined “pediatric” as infants, children, and adolescents less than 18 years of age. The scope of this inquiry includes patients with congenital MM.
OBJECTIVES
The objective of this guideline was to search the current literature, evaluate the evidence and make appropriate recommendations for clinical management of infants with MM closed prenatally vs postnatally, specifically in reference to the incidence of TCS.
METHODS
Writing Group and Question Establishment
The Guidelines Task Force initiated a systematic review of the literature and evidence-based guideline relevant to the diagnosis and treatment of patients with MM. Through objective evaluation of the evidence and transparency in the process of making recommendations, this evidence-based clinical practice guideline was developed for the diagnosis and treatment of patients with MM. These guidelines were developed for educational purposes to assist practitioners in their clinical decision-making processes. Additional information about the methods utilized in this systematic review is provided in the introduction and methodology chapter.
A series of authors for the development of guidelines related to MM were identified and screened for conflict of interest. This group, in turn, agreed on a set of pertinent questions to address the topic at hand, and conducted a systematic review of the literature relevant to MM. The recommendations deliberately eschewed the use of expert opinion, and instead relied strictly on the available literature.
Literature Search
The Guidelines Task Force worked with a research librarian to assist with the formulation of search terms related to SB, MM, TCS and prenatal repair and to develop strategies used to search PubMed and Embase for relevant literature published between 1966 and September 2016. Co-authors used the article inclusion and exclusion criteria described below to screen 261 abstracts and selected 54 relevant articles for full-text review. Staff compiled the results for review and final approval by all the Task Force members. Literature searches of electronic databases were supplemented with manual screenings of the bibliographies of all retrieved publications and other potentially relevant systematic reviews. All literature identified either by searches of the electronic databases or manual searches (15 of the 261 abstracts) were subject to the article inclusion and exclusion criteria listed below. The search strategies are provided within the methods sections of the topics evaluated below.
Study Selection and Eligibility Criteria
The Task Force members collaborated with a medical librarian to search PubMed and Embase for the period from 1966 to September 2016 using the search strategies provided in Appendix I. After de-duplication, the literature search yielded 246 abstracts, which were reviewed by the authors using the following inclusion and exclusion criteria:
- At least 80% of patients had to be patients with MM and <18 years of age.
- Studies that enrolled >20% of patients with other forms of SB were excluded.
- Studies that combined the results of patients with other forms of SB were excluded if the study enrolled less than 80% of target patient population.
- Studies that enrolled mixed patient populations were included only if they reported separate results for the target population. The results of the target population were the only results considered as evidence to support our recommendations.
- The study was a full article report of a clinical study.
- The study was not a meeting abstract, editorial, letter, or a commentary.
- Prospective case series had to report baseline values, if applicable.
- Case series studies with non-consecutive enrollment of patients were excluded.
- Studies had to have appeared in a peer-reviewed publication or a registry report.
- Studies had to enroll at least 10 patients for each distinct outcome measured. If it was a comparative study, a minimum enrollment of 5 patients per treatment arm for each outcome was necessary.
- The study involved humans.
- The study was published between 1966 and September 2016.
- The study presented results quantitatively.
- The study did not involve “in vitro”, “biomechanical” or cadavers.
- The study was published in English.
- Papers reporting results of systematic reviews, meta-analyses, or guidelines developed by others were excluded.
- Authors specifically excluded follow-up studies in which a cohort of patients from an initial study were followed in time and separately reported in a subsequent publication. This prevented the same patients from being included multiple times in this review.
To reduce bias, these criteria were specified before conducting the literature searches. For the purposes of this evidence review, articles that did not meet the selection criteria were not considered potential evidence to support the clinical recommendations. These criteria were also applied to 15 additional articles provided by the Guidelines Task Force who supplemented the electronic database searches with articles from manual searches of the bibliographies of articles yielded by the search.
Three independent reviewers evaluated each abstract to assess if the article was relevant to the question, and results were compared for agreement by a separate party. Inconsistencies were re-reviewed, and disagreements were resolved by consensus. The authors did not include systematic reviews, guidelines, or meta-analyses conducted by others, as these documents were developed using different inclusion criteria than those specified in this guideline. Although these articles were not included as evidence to support the review, they were recalled for full-text review in order for the Guidelines Task Force to conduct manual searches of the bibliographies. Many articles identified in the preliminary search were excluded because they were not specific to MM and included other forms of SB. Studies were only recalled for full review if their abstract suggested that they might address one of the recommendations, and their bibliographies were searched for additional studies. Of the 54 articles selected, 50 were rejected for not meeting inclusion criteria or for being off-topic and 1 was rejected for including the same patients as a subsequent study. There were 3 studies that met inclusion criteria (see Appendix IV).5-7 See PRISMA Article Flow Chart in Appendix II.
Data Collection Process
The abstracts that met the selection criteria mentioned above were retrieved in full-text form. Each article’s adherence to the selection criteria was confirmed. To determine how the data could be classified, the information in the full-text articles was then evaluated to determine whether they were providing results of therapy or were more centered on diagnostic or prognostic information. Agreement on these assessments and on the salient points regarding the type of study design and objectives, and the conclusions and data classification was then reached by exchanging drafts and comments by discussion or e-mail. The information was then used for construction of the evidence tables (see Appendix IV).
Assessment for Risk of Bias
The literature related to MM and prenatal or in utero closure of MM and TCS was assessed. Bias may be a concern when investigators are reporting or tracking outcomes of infants or patients that they have treated. Bias in reporting the incidence of TCS in infants with prenatal repair of MM may occur. Authors attempted to blind the care givers who evaluated these children for motor function and other symptoms in the randomized controlled MOMS trial. There were additional studies that reported a case series of infants with in utero closure of MM infants who were compared to historical controls. One criticism of all studies is that there is limited follow-up data available for infants with prenatal repair for those infants, clinical follow-up over their lifetime and comparison to historical control cohorts would be interesting. The methodological quality of the studies and the risk of bias were assessed using the following 6 criteria:
- Sequence generation
- Allocation concealment
- Blinding
- Incomplete reporting of data
- Selective reporting of outcomes
- Other potential threats to validity
Rating and Classification of the Quality of Evidence
The quality of evidence was rated using an evidence hierarchy for the study type we included for review: therapeutic. Demonstrating the highest degree of clinical certainty, Class I evidence is used to support recommendations of the strongest type, defined as Level I recommendations. Level II recommendations reflect a moderate degree of clinical certainty and are supported by Class II evidence. Level III recommendations denote clinical uncertainty supported by Class III evidence. This hierarchy is shown in Appendix III. Additional information regarding the hierarchy classification of evidence is found here on the CNS Guidelines methodology page.
Revision Plans
In accordance with the Institute of Medicine’s standards for developing clinical practice guidelines, the task force will monitor related publications following the release of this document and will revise the entire document and/or specific sections “if new evidence shows that a recommended intervention causes previously unknown substantial harm; that a new intervention is significantly superior to a previously recommended intervention from an efficacy or harms perspective; or that a recommendation can be applied to new populations.”8 The task force will confirm within 5 years from the date of publication that the content reflects current clinical practice and the available technologies for the incidence of TCS in infants with MM with prenatal vs postnatal repair.
RESULTS
Study Selection and Characteristics
There was Class II evidence from 1 study and Class III evidence from another 2 studies that demonstrated tethered cord syndrome develops in children with prenatal closure of their MM at the same or at a somewhat higher rate than with postnatal closure. There was also an increased risk of development of inclusion cysts in children who underwent in utero closure as fetuses.
Results of Individual Studies, Discussion of Study Limitations and Risk of Bias
In a landmark prospective study, Adzick et al conducted a randomized trial of prenatal versus postnatal repair of MM, and the results were reported in the New England Journal of Medicine (NEJM).5 Primary outcomes evaluated at 12 months were fetal or neonatal death and need for shunt. Evaluators were blinded to initial treatment. Primary outcomes studied and reported at 30 months included mental development, Index of Bayley Scales of Infant Development II and motor function with “adjustment for lesion level”. Radiological and functional levels were compared based on MRI and clinical examination. Secondary outcomes studied were complications of surgery and pregnancy, neonatal morbidity and mortality, time of first shunt placement, locomotion, developmental assessments, Chiari II development, degree of disability, rates of epidermoid cyst development and of spinal cord tethering. Planned enrollment was for 200 patients, however the study was terminated after 183 were randomized. In the first analysis of this cohort, 158 infants had reached the 12 month age timepoint, and 134 were at 30 months.5 All consecutive patients whose mothers met inclusion criteria (Appendix V) and signed an informed consent form were enrolled in the randomized study. Of the 158 infants at 12 months, there were 78 in the prenatal group and 80 infants in the postnatal group. Of the 134 infants at 30 months, there were 64 children in the prenatal group and 70 children in the postnatal group. No further follow-up, after the 30 months, was reported.5 Although this was a Class I study, authors down-graded the class of evidence to Class II because the development of TCS was not the primary outcome studied. However, there was evidence suggesting that spinal cord tethering occurs at a higher rate for infants that had prenatal repair of MM. There was some missing data, so the percentages may not be accurate with a small “n”. While the rate of epidermoid cyst development was similar in both groups, the rate of surgery for tethered cord by 12 months was 8% (6/77) in infants treated prenatally and 1% (1/80) for infants that had their MM repaired after birth. The relative risk was reported as 6.15 (range 0.76 – 50) with a p value of 0.06, so this trend was not statistically significant.
In a Class III retrospective study by Lee et al, it was reported that in utero closure of MM does not improve lower urinary tract function.6 In this study, the primary outcome studied was urological dysfunction. Secondary outcomes reported included the need for ventriculoperitoneal shunt and spinal cord untethering surgery. Prenatal vs postnatal repairs of MM were compared: 11 infants had prenatal repair elsewhere and 22 infants with postnatal repair were chosen from their MM database. These infants were not necessarily consecutive or randomized, but were case matched to historical controls, and may include patients described in the Adzick5 and Danzer7, 9 studies. The mean duration of follow-up was 7.2 years for the prenatal closure group and 7.31 years for the postnatal repair group. Student’s t test or Fisher’s exact test were used to compare cases to controls based on data characteristics. Statistical testing was performed with SAS® 9.2 and a 2-sided p <.05 was considered significant. Lee at al provided evidence that there were no differences between the groups in terms of urological dysfunction. There was no statistical difference in the rate of shunting (p =0.14) or untethering surgery (p =0.99). Three of the 11 prenatal repair infants required detethering (27%) and 6 of the 22 infants that had postnatal repair required detethering (27%) (p = 0.99).6 Although both forms of closure were associated with the development of the need for tethered cord surgery, there were no statistical differences found.
However, in 2008 Danzer et al performed a study that detected a higher incidence of TCS and inclusion cysts in infants with MM repaired in utero.9 In this study, 54 infants were followed on average for 72 months (6 years) +/- 14 months (range 46-98 months).9 The incidence and development of intradural inclusion cysts following prenatal MM closure were studied as the primary outcomes.9 Danzer found that 30% (16/54) of infants repaired prenatally developed symptomatic TCS requiring surgery. While, this rate is similar to the reported range of 10 to 38% for postnatally repaired infants, 63% (10/16) of these infants with TCS had intradural epidermoid inclusion cysts.9 Intradural inclusion cysts have been found less often (<25%) in infants that underwent traditional post-natal repair. This study suggests that infants with MM repaired prenatally may develop symptomatic TCS with inclusion cyst formation earlier than infants repaired postnatally.9
Danzer followed up this study with another (Class III) study reporting long-term results (8-14 years) published in 2016.7 The same 54 patients were included, with a finding that 33% of the children who had prenatal closure (14 patients) developed spinal cord tethering, and of those, 57% involved an intradural dermoid cyst. Of the 14 patients, 11 required only 1 additional surgery, while 2 patients had 2 procedures, and 1 had 4 additional detethering procedures.7 This study provided only Class III evidence because the same patients were reported after longer-term follow-up (with 9 patients lost to follow up and 3 having failed to return the questionnaire), and thus did not really yield any new findings or recommendations. Because Danzer published a second study in 2016, reporting long term follow up data from patients from the 2008 study, only the 2016 study was included as evidence.7
DISCUSSION
Although there are only approximately 1,500 infants born annually with SB and MM in the United States, there are thousands more born each year in developing countries throughout the world, because of folic acid deficiency during conception and pregnancy.1-3 Women carrying fetuses with MM, in the United States, are provided an opportunity to have prenatal repair done for their infants. However, in many countries with limited resources, the care for women carrying fetuses with MM and children with MM may not be as well developed, and children have died with many of the associated comorbidities including Chiari II malformation, HC, neurogenic bowel and bladder syndrome and spinal deformities.
The 1999 JAMA study, published by Bruner and Tulipan et al first reported the outcomes for infants treated with fetal surgery for MM.4 They demonstrated a significantly decreased incidence of shunt-dependent hydrocephalus and a reduced incidence of Chiari II development.4 However, the median follow-up was only 311 days (range 182-799) and the development of TCS was not an outcome studied.4 In a 2002 NEJM report, Mazzola et al reported 3 cases of infants with MM that were repaired prenatally; in all 3 cases, TCS developed secondary to large intradural inclusion cysts.10 This study did not meet inclusion criteria because of the small number of patients reported.
There was another study, excluded for low study population number, which also reported a similar incidence of TCS in these infants closed prenatally, as compared to infants closed after birth.11 In Farmer’s early 2003 report, 13 infants with MM were repaired prenatally. However, only 9 survived at 1 year for a retrospective review.11 This was a single-center, retrospective case series with an average of 17 months’ follow-up. One infant required surgery for TCS at 15 months of age and the mortality rate in their series was 31% (4/13). Although this paper did not indicate an increased rate of TCS, it did report a single case of early TCS in an infant that underwent MM repair in-utero.11
This systematic review of the literature revealed a paucity of good, long-term clinical data. Perhaps because the technique of in-utero closure is relatively “new”, we do not yet have the numbers of randomized patients with MM that could demonstrate a significant difference in the rate of TCS associated with either closure technique. This review did show some early Class II and Class III studies that demonstrated that the risk of development of TCS in infants closed in-utero may be similar to or higher than infants closed postnatally. Additionally, in 2008, Danzer evaluated 54 infants who had prenatal repair of their MM and assessed them for the development of TCS (prior to initiation of the MOMS trial).9 The authors reported a 30% incidence of TCS (16/54) in these infants, which developed at a median age of 27 months.9 It was also noted that in 10 of the 16 children (63%), TCS was associated with or caused by an intradural inclusion cyst (dermoid or epidermoid).9 Adzick et al reported in 2011, in NEJM, that there was a higher trend of TCS development in infants with prenatal repair and that there were more inclusion cysts seen, but neither increase was found to be significant.5 In 2016, Danzer reported long-term outcomes of 42 of the above 54 patients, again noting a slight increased risk of the development of inclusion cysts, causing TCS in infants with prenatal repair.7
Differences between prenatal and postnatal repair with respect to the development of TCS and inclusion cysts should be considered alongside other relevant maternal and fetal outcomes when deciding upon a preferred method of MM closure.
Future Research
This guideline highlights the need for increased surveillance for the development of inclusion cysts and TCS in children who have had MM repair performed in utero. Based on the studies included as evidence, clinical and radiological follow up of all children with MM should be reported again in the future. Additionally, infants who undergo this procedure in the future should be carefully monitored. Relative risks of prenatal closure should be studied, and risk-benefit ratios should be carefully considered by parents and healthcare providers of infants with MM diagnosed prenatally. It may be that the relatively small size of the MM infants closed in utero makes the closure technique more difficult. With the small size, thinner skin, less subcutaneous fat and perhaps thinner dura, the risk of accidentally including a small part of epidermis or dermis in the closure may be slightly higher. The development of inclusion cysts may be related to the utilization of various biomembranes in the closure techniques. Inclusion cysts may also be a natural part of the maldevelopment of the terminal neural tube. As time goes by, there will be an ever-increasing number of patients to evaluate and follow. Continued review and assessment of long-term clinical function of these patients will be important in the future.
CONCLUSIONS
In conclusion, although there is a paucity of data, there does seem to be some Class II and III evidence indicating the same or a slightly higher rate of TCS and/or inclusion cyst development in children who had in utero closure of their MM. Additionally, TCS seems to develop at an earlier age in such infants. Increased surveillance for TCS and/or inclusion cysts is therefore warranted.
Conflict of Interest
The Guidelines Task Force members were required to report all possible conflicts of interest (COIs) prior to beginning work on the guideline, using the COI disclosure form of the AANS/CNS Joint Guidelines Review Committee, including potential COIs that are unrelated to the topic of the guideline. The CNS Guidelines Committee and Guideline Task Force Chair reviewed the disclosures and either approved or disapproved the nomination. The CNS Guidelines Committee and Guideline Task Force Chair are given latitude to approve nominations of Task Force Members with possible conflicts and address this by restricting the writing and reviewing privileges of that person to topics unrelated to the possible COIs. The conflict of interest findings are provided in detail in the companion introduction and methods manuscript.
Disclosures
These evidence-based clinical practice guidelines were funded exclusively by the Congress of Neurological Surgeons, which received no funding from outside commercial sources to support the development of this document.
Disclaimer of Liability
This clinical systematic review and evidence-based guideline was developed by a multidisciplinary physician volunteer task force and serves as an educational tool designed to provide an accurate review of the subject matter covered. These guidelines are disseminated with the understanding that the recommendations by the authors and consultants who have collaborated in their development are not meant to replace the individualized care and treatment advice from a patient's physician(s). If medical advice or assistance is required, the services of a competent physician should be sought. The proposals contained in these guidelines may not be suitable for use in all circumstances. The choice to implement any particular recommendation contained in these guidelines must be made by a managing physician in light of the situation in each particular patient and on the basis of existing resources.
ACKNOWLEDGMENTS
The guidelines task force would like to acknowledge the Congress of Neurological Surgeons Guidelines Committee for their contributions throughout the development of the guideline, the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Guidelines Review Committee, as well as the American Academy of Pediatrics, Child Neurology Society and Spina Bifida Association for their review, comments, and suggestions throughout peer review, as well as the contributions of Trish Rehring, MPH, CHES, Senior Manager of Clinical Practice Guidelines for the CNS, and Gretchen Kuntz, MSW, MLIS, for assistance with the literature searches. Throughout the review process, the reviewers and authors were blinded from one another. At this time the guidelines task force would like to acknowledge the following individual peer reviewers for their contributions: Kimon Bekelis, MD; Robin Bowman, MD; Timothy J. Brei, MD; Andrew P. Carlson, MD; John Chi, MD; Mark Dias, MD; Jeffrey Olson, MD; John O’Toole, MD; Michael Partington, MD; Curtis Rozzelle, MD; Krystal Tomei, MD; Jan B. Wollack, MD, PhD.
Appendix I. Literature Search Terms
| PubMed Strategy |
Results |
Embase Strategy |
Results |
Total Results after De-duplication |
| (((((((spina bifida[mh] OR spina bifida[tw])) OR (myelomeningocele[mh] OR myelomeningocele[tw]))) AND (("Neural Tube Defects"[mh]) OR "tethered cord syndrome" [tw]))) AND ((("in utero closure"[tw]) OR "post-natal"[tw]) OR "in utero"[tw])) NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms]) |
153 |
(('neural tube defect'/exp OR 'tethered cord syndrome') AND ('in utero closure' OR 'post-natal' OR 'in utero') AND (('meningomyelocele'/exp OR meningomyelocele) OR ('spinal dysraphism'/exp OR 'spina bifida'))) AND 'human'/de AND [embase]/lim NOT [medline]/lim |
95 |
246 |
Appendix II. PRISMA Flow Chart for the literature search for the incidence of TCS in infants with prenatal versus postnatal repair
Appendix III: Rating Evidence Quality
Classification of Evidence on Therapeutic Effectiveness
| Class I Evidence Level I Recommendation |
Evidence from one or more well-designed, randomized controlled clinical trial, including overviews of such trials. |
| Class II Evidence Level II Recommendation |
Evidence from one or more well-designed comparative clinical studies, such as non-randomized cohort studies, case-control studies, and other comparable studies, including less well-designed randomized controlled trials. |
| Class III Evidence Level III Recommendation |
Evidence from case series, comparative studies with historical controls, case reports, and expert opinion, as well as significantly flawed randomized controlled trials. |
Appendix IV. Evidence Tables
| Article (Author, Year) |
Class of Evidence |
Summary |
Conclusions |
|
Adzick NS, 20115
|
Class II |
Randomized prospective trial of prenatal vs postnatal closure with outcomes at 30 months of age reported for 134/183 enrolled. Class II evidence for this topic as tethered cord and dermal inclusion cysts analyzed as secondary outcomes. Difference did not reach statistical significance. |
This paper provides evidence that spinal cord tethering occurs at a higher rate for infants that had prenatal repair of myelomeningocele. While the rate of epidermoid cyst development was similar in both groups, the rate of surgery for tethered cord by 12 months was 8% (6/77) in infants treated prenatally and 1% (1/80) for infants that had their myelomeningocele repaired after birth. The relative risk was 6.15 (range 0.76 – 50) with a p value of .06, so this trend was not significant. In conclusion, there was an increased risk of tethered cord for infants closed in utero, but the trend was not statistically significant. |
|
Lee NG, 20126
|
Class III |
Retrospective historical matched control group of postnatal closure (n=22, 7.3 +/- 4.2 years follow-up) to prenatal closure patients (n=11, 7.2 +/- 4.2 years follow up). Primary objective was assessing urologic function, also looked at incidence of tethered cord surgery. |
This paper provides evidence that: There were no differences between the groups in terms of urological function. There was no difference in the rate of shunting (p = 0.14) or untethering surgery (p = 0.99). Three of the 11 prenatal repair infants required detethering (27%) and 6 of the 22 infants that had postnatal repair required detethering (27%) (p = 0.99). In conclusion, there was no difference in the rate of TCS development. |
|
Danzer E, 20167
|
Class III |
Longer term (8-14 years) follow up of 42 out of an initial 54 patients with prenatal closure prior to MOMS study. |
Danzer followed his 2008 study with a Class III study reporting long-term results: 33% of the children who had prenatal closure (14 patients) developed spinal cord tethering, with and of those, 57% developed an intradural dermoid cyst. |
Appendix V. Major Inclusion/Exclusion Criteria of the MOMS12 Trial
| Inclusion |
- Mothers >18 years of age carrying a singleton pregnancy
- Fetuses with normal karyotype
- Fetuses with a gestational age of 19-25.9 weeks at randomization
- Fetuses with MM defect with an upper level between T1 and S1 with evidence of hindbrain herniation
|
| Exclusion |
- Fetuses with a severe kyphotic deformity related to MM
- Fetuses with a fetal anomaly unrelated to MM
- Risk of preterm birth, placental abruption or any other contraindication to surgery.
- Mother with Body Mass Index of >35
|
REFERENCES
- Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res. A Clin. Mol. Teratol. Dec 2010;88(12):1008-1016.
- Mburia-Mwalili A, Yang W. Birth Defects Surveillance in the United States: Challenges and Implications of International Classification of Diseases, Tenth Revision, Clinical Modification Implementation. Int Sch Res Notices. 2014;2014:212874.
- Atta CA, Fiest KM, Frolkis AD, et al. Global Birth Prevalence of Spina Bifida by Folic Acid Fortification Status: A Systematic Review and Meta-Analysis. Am. J. Public Health. Jan 2016;106(1):e24-34.
- Bruner JP, Tulipan N, Paschall RL, et al. Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA. Nov 17 1999;282(19):1819-1825.
- Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N. Engl. J. Med. Mar 17 2011;364(11):993-1004.
- Lee NG, Gomez P, Uberoi V, et al. In utero closure of myelomeningocele does not improve lower urinary tract function. J. Urol. Oct 2012;188(4 Suppl):1567-1571.
- Danzer E, Thomas NH, Thomas A, et al. Long-term neurofunctional outcome, executive functioning, and behavioral adaptive skills following fetal myelomeningocele surgery. Am. J. Obstet. Gynecol. Feb 2016;214(2):269.e261-269.e268.
- Ransohoff DF, Pignone M, Sox HC. How to decide whether a clinical practice guideline is trustworthy. JAMA. Jan 9 2013;309(2):139-140.
- Danzer E, Adzick NS, Rintoul NE, et al. Intradural inclusion cysts following in utero closure of myelomeningocele: clinical implications and follow-up findings. J. Neurosurg. Pediatr. Dec 2008;2(6):406-413.
- Mazzola CA, Albright AL, Sutton LN, Tuite GF, Hamilton RL, Pollack IF. Dermoid inclusion cysts and early spinal cord tethering after fetal surgery for myelomeningocele. N. Engl. J. Med. Jul 25 2002;347(4):256-259.
- Farmer DL, von Koch CS, Peacock WJ, et al. In utero repair of myelomeningocele: experimental pathophysiology, initial clinical experience, and outcomes. Arch. Surg. Aug 2003;138(8):872-878.