Local and Systemic Therapy of Recurrent Ependymoma in Children and Adolescents: Short- and Long-term Results of the E-HIT-REZ 2005 Study
- The treatment of ependymoma has traditional been limited to surgical resection in isolation as radiation and chemotherapy display limited efficacy in improving progression-free and overall survival.
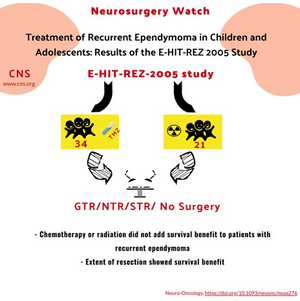
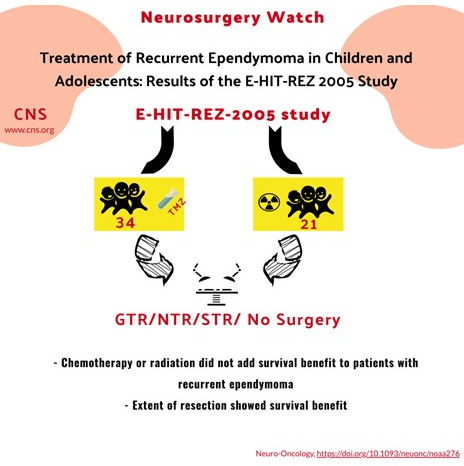
- The goal of this study was to assess the German multi-center E-HIT-REZ-2005 study for efficacy of radiation and chemotherapy in adolescent and pediatric ependymoma patients at 1st recurrence.
- The study enrolled patient from 2006 – 2013 and enrolled a total of 53 patients with a median age of 6.9 years (range: 4-14 years) representing 45% of the patient population in Germany during this time period treated for ependymoma at 1st recurrence.
- Median overall survival in the enrolled patient population was 36 months with a 5-year overall survival of 37% +-66%.
- 21 patients received temozolomide prior to re-resection and 18 (85.7%) displayed progression at a median of 1 month after treatment onset.
- 21 patients received radiation prior to re-resection and median PFS was similar between the radiated group at 21 months compared to the non-irradiated group at 19 months and median OS was equivalent between groups at 36 months.
- 37 patients in the study that received re-resection at the time of 1st Patients with GTR/NTR displayed a significantly increased PFS at 25.9 months (CI:15.0-36.6) and OS at 71 months (CI:12.7-129.3) as compared to patients with subtotal resection with PFS at 9.2 months (CI: 7.2-23) and OS at 20.6 months (CI:3.8-37.4).
- Multivariate Cox regression analysis revealed that GTR/NTR displayed the largest reduction in risk of death.
- While the patient cohort is relatively small, this multi-center study further validates the limited efficacy of chemotherapy and radiation in patients with ependymoma and the importance of maximum safe surgical resection.
Source
New England Journal of Medicine